Back
Poster Session C
Vasculitis
Session: (1543–1578) Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
1566: Outcome of Vascular Involvement of Behçet Syndrome Treated with Infliximab: A Retrospective Cohort Study
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NT
Nur Beyza Tukek, MD
Istanbul University Cerrahpasa - Cerrahpasa Medical School
ISTANBUL, Turkey
Abstract Poster Presenter(s)
Gulen Hatemi1, Nur Beyza Tukek2, Sinem Nihal Esatoglu1, Yesim Ozguler3, Sitki Safa Taflan2, Melike Melikoglu1, Serdal Ugurlu4, Izzet Fresko1, Zekayi Kutlubay5, Sebahattin Yurdakul1, Hasan Yazici6 and Vedat Hamuryudan7, 1Istanbul University-Cerrahpaşa, Cerrahpasa Medical Faculty, Department of Internal Medicine, Division of Rheumatology, Istanbul, Turkey, 2Istanbul University-Cerrahpaşa, Cerrahpasa Medical Faculty, Department of Internal Medicine, Istanbul, Turkey, 3Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, Department of Internal Medicine, Division of Rheumatology, New York, NY, 4Istanbul University-Cerrahpaşa, Istanbul, Turkey, 5Istanbul University-Cerrahpaşa, Cerrahpasa Medical Faculty, Department of Dermatology, Istanbul, Turkey, 6Letter to Editor Rheumatology, Istanbul, Turkey, 7Istanbul University-Cerrahpaşa, Cerrahpaşa Medical School, Department of Internal Medicine, Division of Rheumatology, Istanbul, Turkey
Background/Purpose: Vascular involvement is an important cause of morbidity and mortality in patients with Behçet syndrome (BS). We aimed to survey the efficacy and safety of infliximab (IFX) in BS patients with vascular involvement followed in a dedicated tertiary center.
Methods: Charts of all BS patients who used IFX for vascular involvement between 2004-2021 were reviewed. Primary endpoint was remission at month 6, defined as no new clinical symptoms/findings associated with a vascular lesion, no worsening of the primary vascular lesion, no new vascular lesions on imaging, and CRP< 10 mg/dL. For patients with venous ulcers, remission was defined as the total healing of the existing venous ulcers. Relapse was defined as development of a new vascular lesion or recurrence of a preexisting vascular lesion.
Results: Among the 127 patients (102 men, mean age at IFX initiation: 35.8 ± 9 years) treated with IFX, 110 (87%) received IFX for remission induction of whom 87 (79%) being refractory to conventional immunosuppressives and 17 received IFX for maintenance therapy. Overall, remission rate was 73% (93/127) at month 6 and 63% (80/127) at month 12. For subgroups,the remission rate was 82% (50/61) and 70% (43/61) among patients with venous thrombosis, 84% (31/37) and 68% (25/37) among those with pulmonary artery involvement, 69% (11/16) and 63% (10/16) among those with non-pulmonary artery involvement, and 8% (1/13) and 15% (2/13) among those with venous ulcers at month 6 and at month 12, respectively. 17/100 (17%) patients experienced 22 relapsesduring a mean follow-up of 28.4 ± 21 months of IFX therapy.Among the 22 relapses, 12 were progression of a pre-existing vascular lesion and 10 were new vascular lesions. Fourteen patients discontinued IFX due to adverse events. These were: allergic reactions (n=5), infections (n=3), malignancy (n=2), paradoxical reactions (n=3), anda heart failure. Four patients had died the reasons being lung adenocarcinoma, sepsis, and pulmonary hypertension related right heart failure due to pulmonary artery thrombosis (n=2), respectively.
Conclusion: Infliximab seems to be highly effective in BS patients with venous thrombosis and pulmonary artery involvement even among those who are refractory to immunosuppressives and glucocorticoids. .The remission rate was somewhat lower among patients with non-pulmonary artery involvement and no remission could be achieved in the majority of patients with venous ulcers.
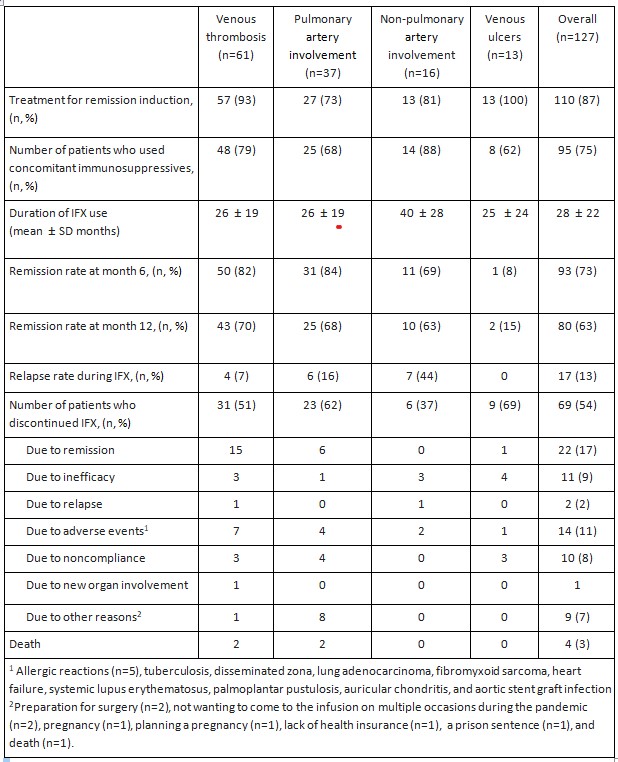 Table 1. The frequency of concomitant immunosuppressive use, duration of infliximab use and outcomes of BS patients with vascular involvement treated with IFX
Table 1. The frequency of concomitant immunosuppressive use, duration of infliximab use and outcomes of BS patients with vascular involvement treated with IFX
Disclosures: g. Hatemi, Celgene, UCB, Novartis, AbbVie/Abbott; N. Tukek, None; S. Esatoglu, None; Y. Ozguler, UCB, Novartis, Pfizer; S. Taflan, None; M. Melikoglu, None; S. Ugurlu, None; I. Fresko, None; Z. Kutlubay, None; S. Yurdakul, None; H. Yazici, None; V. Hamuryudan, Celgene, AbbVie/Abbott, Novartis, UCB.
Background/Purpose: Vascular involvement is an important cause of morbidity and mortality in patients with Behçet syndrome (BS). We aimed to survey the efficacy and safety of infliximab (IFX) in BS patients with vascular involvement followed in a dedicated tertiary center.
Methods: Charts of all BS patients who used IFX for vascular involvement between 2004-2021 were reviewed. Primary endpoint was remission at month 6, defined as no new clinical symptoms/findings associated with a vascular lesion, no worsening of the primary vascular lesion, no new vascular lesions on imaging, and CRP< 10 mg/dL. For patients with venous ulcers, remission was defined as the total healing of the existing venous ulcers. Relapse was defined as development of a new vascular lesion or recurrence of a preexisting vascular lesion.
Results: Among the 127 patients (102 men, mean age at IFX initiation: 35.8 ± 9 years) treated with IFX, 110 (87%) received IFX for remission induction of whom 87 (79%) being refractory to conventional immunosuppressives and 17 received IFX for maintenance therapy. Overall, remission rate was 73% (93/127) at month 6 and 63% (80/127) at month 12. For subgroups,the remission rate was 82% (50/61) and 70% (43/61) among patients with venous thrombosis, 84% (31/37) and 68% (25/37) among those with pulmonary artery involvement, 69% (11/16) and 63% (10/16) among those with non-pulmonary artery involvement, and 8% (1/13) and 15% (2/13) among those with venous ulcers at month 6 and at month 12, respectively. 17/100 (17%) patients experienced 22 relapsesduring a mean follow-up of 28.4 ± 21 months of IFX therapy.Among the 22 relapses, 12 were progression of a pre-existing vascular lesion and 10 were new vascular lesions. Fourteen patients discontinued IFX due to adverse events. These were: allergic reactions (n=5), infections (n=3), malignancy (n=2), paradoxical reactions (n=3), anda heart failure. Four patients had died the reasons being lung adenocarcinoma, sepsis, and pulmonary hypertension related right heart failure due to pulmonary artery thrombosis (n=2), respectively.
Conclusion: Infliximab seems to be highly effective in BS patients with venous thrombosis and pulmonary artery involvement even among those who are refractory to immunosuppressives and glucocorticoids. .The remission rate was somewhat lower among patients with non-pulmonary artery involvement and no remission could be achieved in the majority of patients with venous ulcers.
 Table 1. The frequency of concomitant immunosuppressive use, duration of infliximab use and outcomes of BS patients with vascular involvement treated with IFX
Table 1. The frequency of concomitant immunosuppressive use, duration of infliximab use and outcomes of BS patients with vascular involvement treated with IFXDisclosures: g. Hatemi, Celgene, UCB, Novartis, AbbVie/Abbott; N. Tukek, None; S. Esatoglu, None; Y. Ozguler, UCB, Novartis, Pfizer; S. Taflan, None; M. Melikoglu, None; S. Ugurlu, None; I. Fresko, None; Z. Kutlubay, None; S. Yurdakul, None; H. Yazici, None; V. Hamuryudan, Celgene, AbbVie/Abbott, Novartis, UCB.

