Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1417–1439) RA – Treatment Poster III
1420: Outcomes in Patients with Rheumatoid Arthritis Initiating Monotherapy with Etanercept, Adalimumab, or Janus Kinase Inhibitors
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
- DP
Dimitrios Pappas, MD, MPH
CorEvitas
New York, NY, United States
Abstract Poster Presenter(s)
Dimitrios Pappas1, Jacqueline O'Brien2, Mike Kelleman2, Lin Guo2, Ying Shan2, Joshua Baker3, Greg Kricorian4, Scott Stryker5 and David Collier6, 1Corrona Research Foundation; CorEvitas, LLC, Albany, NY, 2CorEvitas, LLC, Waltham, MA, 3University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, 4Amgen, Inc., Thousand Oaks, CA, 5Amgen Inc., San Francisco, CA, 6Amgen Inc., Simi Valley, CA
Background/Purpose: Biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) often are used as monotherapy in rheumatoid arthritis (RA). However, little is known about the comparative effectiveness of these therapies when used as monotherapy. In this real-world study, we evaluated first-line use of etanercept (ETN), adalimumab (ADA), and Janus kinase inhibitors (JAKis) as monotherapies to gain insights into drug persistency and effectiveness in a population of monotherapy initiators.
Methods: Data were obtained from the prospective, multicenter, observational, disease-based CorEvitas RA Registry. The study population included b/tsDMARD-naive patients who initiated first-line ETN, ADA, or JAKis as monotherapy. We report descriptive statistics at baseline, persistency on therapy, details on patterns of drug switching, and effectiveness outcomes presented as unadjusted mean change and results from linear and logistic mixed-effects regression models adjusted for covariates that were imbalanced (standardized difference >0.1) at baseline. Outcomes were evaluated at 6 and 12 months.
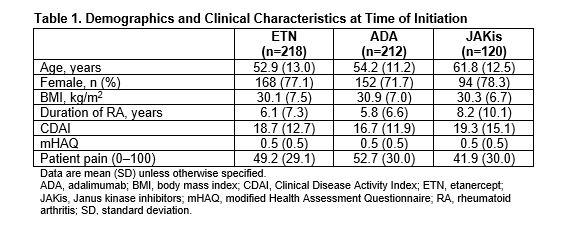
Results: There were 550 initiators of first-line b/tsDMARD monotherapy with a follow-up visit (ETN: 218; ADA: 212; JAKis: 120). First-line JAKi initiators were older and had longer disease duration than ETN and ADA initiators (Table 1). Initiators of ETN and JAKis had slightly higher Clinical Disease Activity Index (CDAI) at baseline than initiators of ADA (Table 1). At 6 months, 64.5%, 65.4%, and 62.4% of initiators remained on ETN, ADA, and JAKis, respectively. At 12 months, 50.7%, 57.3%, and 48.1% remained on ETN, ADA, and JAKis, respectively. At 6 months, 9.6% of ETN initiators, 12.0% of ADA initiators, and 15.4% of JAKi initiators switched to another b/tsDMARD. At 12 months, 22.8% of ETN initiators, 25.2% of ADA initiators, and 20.8% of JAKi initiators switched to another b/tsDMARD. Unadjusted changes in disease activity measures and patient-reported outcomes were generally similar among groups at 6 and 12 months (Table 2). After adjusting for baseline covariates, differences in changes in disease activity measures and patient-reported outcomes between groups were not significant (Figure 1). Regression coefficients (95% CI) for adjusted change in CDAI, patient pain, and modified Health Assessment Questionnaire in ADA initiators relative to ETN initiators were −0.84 (−3.31, 1.62), 0.75 (−5.09, 6.60), and −0.03 (−0.11, 0.05) at 6 months. Regression coefficients for these measures in JAKi initiators relative to ETN initiators were 0.67 (−2.22, 3.56), 4.32 (−2.52, 11.15), and 0.04 (−0.05, 0.13). Odds ratios (95% CI) for achievement of low disease activity at 6 months relative to ETN initiators (after adjusting for imbalanced baseline covariates) were 0.88 (0.49, 1.59) for ADA initiators and 1.04 (0.50, 2.16) for JAKi initiators. Adjusted results were similar at 6 and 12 months.
Conclusion: In a population of patients with RA initiating their first b/tsDMARD as monotherapy, we did not observe differences in 6- and 12-month effectiveness outcomes between ETN, ADA, and JAKi monotherapy initiators after adjusting for baseline differences between groups.
.jpg)
.jpg)
Disclosures: D. Pappas, CorEvitas, LLC, Corrona Research Foundation, AbbVie, Novartis, Roche Hellas, Sanofi; J. O'Brien, CorEvitas, LLC.; M. Kelleman, CorEvitas, LLC.; L. Guo, CorEvitas, LLC.; Y. Shan, CorEvitas, LLC.; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; G. Kricorian, Amgen; S. Stryker, Amgen; D. Collier, Amgen.
Background/Purpose: Biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) often are used as monotherapy in rheumatoid arthritis (RA). However, little is known about the comparative effectiveness of these therapies when used as monotherapy. In this real-world study, we evaluated first-line use of etanercept (ETN), adalimumab (ADA), and Janus kinase inhibitors (JAKis) as monotherapies to gain insights into drug persistency and effectiveness in a population of monotherapy initiators.
Methods: Data were obtained from the prospective, multicenter, observational, disease-based CorEvitas RA Registry. The study population included b/tsDMARD-naive patients who initiated first-line ETN, ADA, or JAKis as monotherapy. We report descriptive statistics at baseline, persistency on therapy, details on patterns of drug switching, and effectiveness outcomes presented as unadjusted mean change and results from linear and logistic mixed-effects regression models adjusted for covariates that were imbalanced (standardized difference >0.1) at baseline. Outcomes were evaluated at 6 and 12 months.
Results: There were 550 initiators of first-line b/tsDMARD monotherapy with a follow-up visit (ETN: 218; ADA: 212; JAKis: 120). First-line JAKi initiators were older and had longer disease duration than ETN and ADA initiators (Table 1). Initiators of ETN and JAKis had slightly higher Clinical Disease Activity Index (CDAI) at baseline than initiators of ADA (Table 1). At 6 months, 64.5%, 65.4%, and 62.4% of initiators remained on ETN, ADA, and JAKis, respectively. At 12 months, 50.7%, 57.3%, and 48.1% remained on ETN, ADA, and JAKis, respectively. At 6 months, 9.6% of ETN initiators, 12.0% of ADA initiators, and 15.4% of JAKi initiators switched to another b/tsDMARD. At 12 months, 22.8% of ETN initiators, 25.2% of ADA initiators, and 20.8% of JAKi initiators switched to another b/tsDMARD. Unadjusted changes in disease activity measures and patient-reported outcomes were generally similar among groups at 6 and 12 months (Table 2). After adjusting for baseline covariates, differences in changes in disease activity measures and patient-reported outcomes between groups were not significant (Figure 1). Regression coefficients (95% CI) for adjusted change in CDAI, patient pain, and modified Health Assessment Questionnaire in ADA initiators relative to ETN initiators were −0.84 (−3.31, 1.62), 0.75 (−5.09, 6.60), and −0.03 (−0.11, 0.05) at 6 months. Regression coefficients for these measures in JAKi initiators relative to ETN initiators were 0.67 (−2.22, 3.56), 4.32 (−2.52, 11.15), and 0.04 (−0.05, 0.13). Odds ratios (95% CI) for achievement of low disease activity at 6 months relative to ETN initiators (after adjusting for imbalanced baseline covariates) were 0.88 (0.49, 1.59) for ADA initiators and 1.04 (0.50, 2.16) for JAKi initiators. Adjusted results were similar at 6 and 12 months.
Conclusion: In a population of patients with RA initiating their first b/tsDMARD as monotherapy, we did not observe differences in 6- and 12-month effectiveness outcomes between ETN, ADA, and JAKi monotherapy initiators after adjusting for baseline differences between groups.

.jpg)
.jpg)
Disclosures: D. Pappas, CorEvitas, LLC, Corrona Research Foundation, AbbVie, Novartis, Roche Hellas, Sanofi; J. O'Brien, CorEvitas, LLC.; M. Kelleman, CorEvitas, LLC.; L. Guo, CorEvitas, LLC.; Y. Shan, CorEvitas, LLC.; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; G. Kricorian, Amgen; S. Stryker, Amgen; D. Collier, Amgen.

