Poster Session A
Systemic lupus erythematosus (SLE)
0371: A Phase 2, Double-blind, Randomized, Placebo-Controlled Multicenter Study to Evaluate Efficacy, Safety, and Tolerability of JBT-101/Lenabasum in Systemic Lupus Erythematosus, an Autoimmunity Centers of Excellence Study (ALE09)
- MM
Meggan Mackay, MD, MS
Feinstein Institutes for Medical Research
Manhasset, NY, United States
Abstract Poster Presenter(s)
Background/Purpose: Musculoskeletal (MSK) symptoms are common in SLE, often associate with debilitating pain requiring immunosuppressive treatment. JBT-101 is a non-psychotropic, non-immunosuppressive, cannabinoid receptor 2 agonist that stimulates resolution of inflammation by increased production of specialized pro-resolving mediators and decreased production of inflammatory molecules. Our goal was to test efficacy and safety of JBT-101 for treatment of inflammatory MSK disease in SLE.
Methods: Adults (18-70 years) meeting SLE ACR criteria with active MSK symptoms (SLEDAI arthritis or BILAG 2004 mild/moderate arthritis), on stable SLE medications and prednisone ≤10 mg daily, with average maximum daily pain scores ≥ 4 on a 10-point numerical rating scale (NRS), were randomized to 12 weeks treatment with placebo (PBO), or 10mg (low), 20mg (med), or 40mg (high) daily of JBT-101. NRS scores were reported daily. Disease activity was assessed at Days 1, 29, 57, 84 and 113; these analyses are pending. The primary endpoint, improvement in NRS score from Days 1 to 84, was analyzed with a mixed effects regression model, that included the screening 7 day average pain NRS and Fibromyalgia Symptom Scale (FSS) scores as covariates to estimate longitudinal trends in NRS and change over time for each treatment group. NRS data up to last day of study treatment were included except days when narcotic analgesics were taken. A secondary endpoint, pain category, was defined using the average 7 day NRS prior to study visits: ≤ 1 no pain, ( >1- ≤3) mild, ( >3-≤7) moderate, >7 severe.
Results: The 101 randomized and treated subjects (Table 1) had a mean screening 7 day average pain NRS=6.6 and mean FSS score of 15.7. FSS scores ≥13 are consistent with a fibromyalgia diagnosis; 71.3% had FSS scores ≥13. The covariate-adjusted estimated change in NRS from Days 1 to 84 was not significantly different between groups: -0.3 PBO, -0.9 low, -1.5 med, -1.2 high (p=.419) (Fig 1). Pairwise-comparisons of active arms versus PBO were not significant (all p >.3, Bonferroni corrected). Among 84 subjects with NRS data at the end of the treatment period at Day 85, pain improved by at least 1 pain category from baseline for 3 (14%) PBO, 10 (45%) low dose, 8 (38%) med dose, and 9 (47%) high dose subjects (p=0.083 overall, Cochran-Mantel-Haenszel C2; p=0.012 pooled active vs PBO, Pearson's C2.).
No grade 4 or 5 adverse events (AEs) were observed (Table 2). 75 subjects experienced at least 1 grade 2 or 3 AE: 20 (80%) PBO, 19 (79%) low, 21 (78%) mod, 15 (60%) high. 5 subjects experienced treatment-related AEs resulting in study drug discontinuation: 1 (4%) med, 4 (16%) high, all were Grade 2. 6 subjects experienced serious AEs: 2 (8%) PBO, 3 (11%) med, 1 (4%) high; all unrelated to study drug. 2 subjects in each active arm experienced severe disease flares (excluding MSK domain), compared to 3 in PBO.
Conclusion: JBT-101 was safe and well-tolerated. While the pre-specified mixed effects model did not identify significant differences between changes in NRS pain scores in active arms vs. PBO, the frequency of pain category improvement at Day 84 was notably higher in the active arms vs PBO. Further analyses, including those of SLEDAI, BILAG and PGA, may identify patients most likely to respond to JBT-101. NCT03093402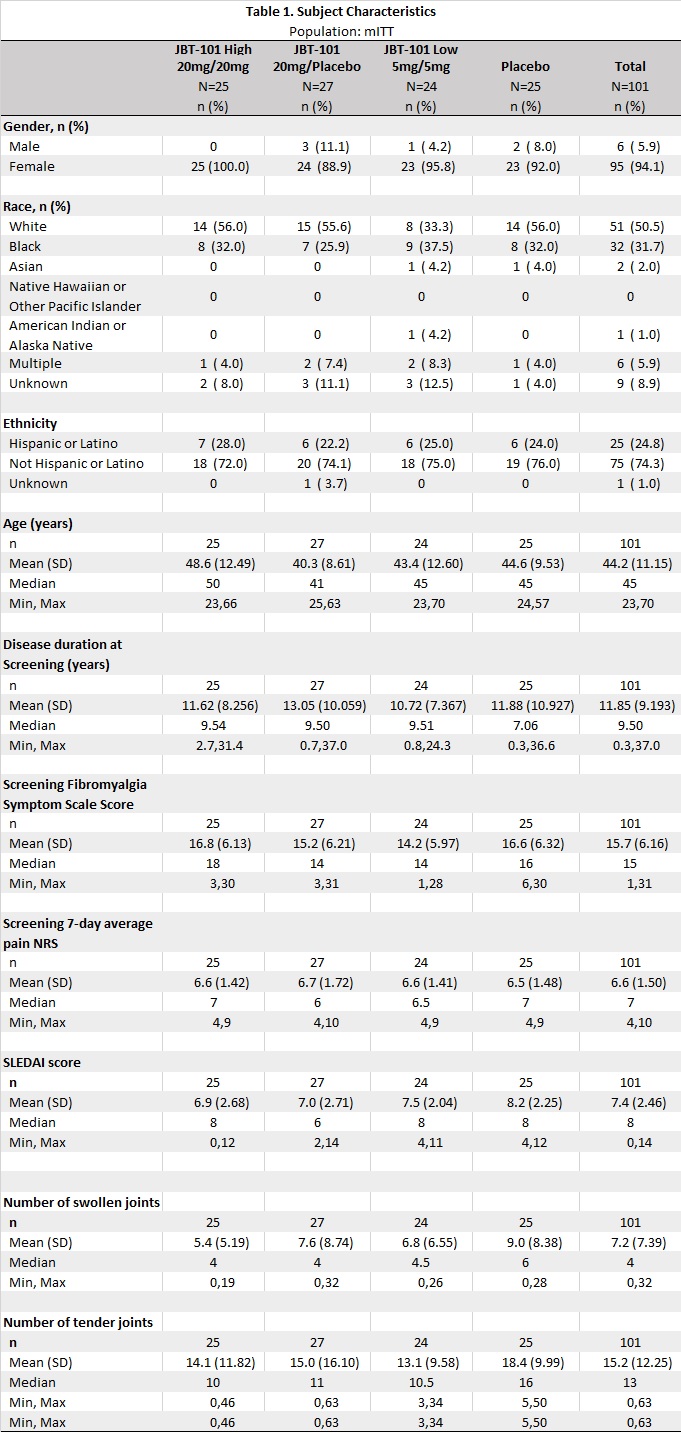
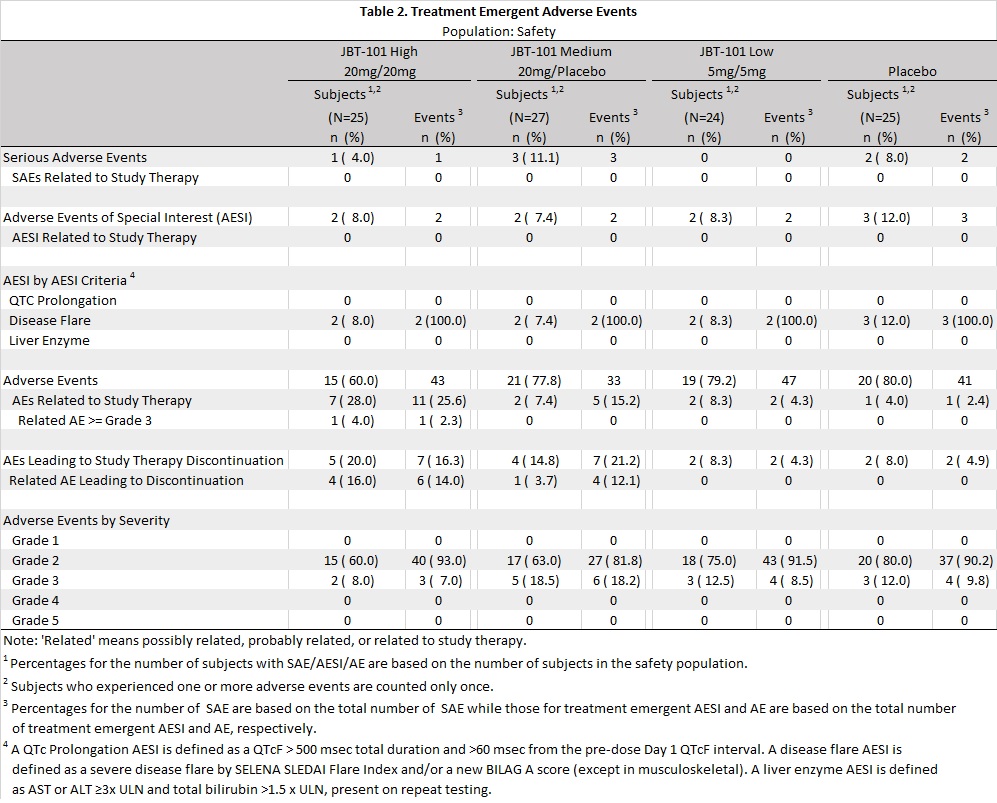
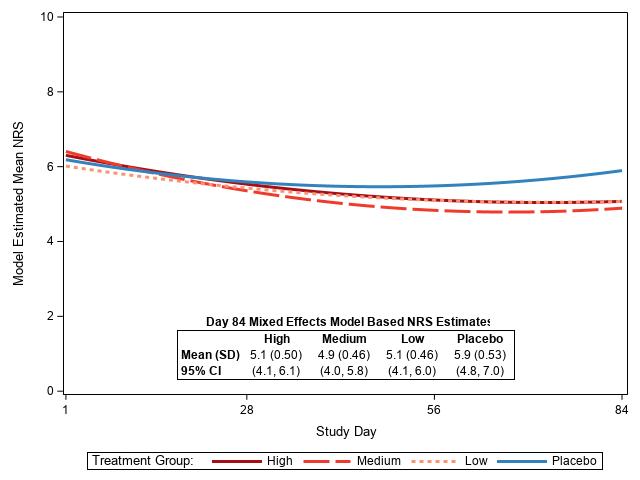 Figure 1. Change in Numerical Rating Scale (NRS) scores for pain over time. Daily NRS scores were analyzed with a mixed effects regression model, that included the screening 7-day average pain NRS and Fibromyalgia Symptom Scale (FSS) scores as covariates.
Figure 1. Change in Numerical Rating Scale (NRS) scores for pain over time. Daily NRS scores were analyzed with a mixed effects regression model, that included the screening 7-day average pain NRS and Fibromyalgia Symptom Scale (FSS) scores as covariates.
Disclosures: M. Mackay, None; R. Zurier, Corbus Pharmaceuticals; D. Kamen, None; F. Koumpouras, AstraZeneca, GlaxoSmithKlein(GSK), aurinia; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Schulz, None; G. Franchin, Eli Lilly, GlaxoSmithKlein(GSK), Sanofi, Pfizer; N. Olsen, None; A. Coca, None; R. Caricchio, None; M. McMahon, None; M. Dall'Era, GlaxoSmithKline (GSK), AstraZeneca, Aurinia, BMS, Amgen; A. Saxena, Eli Lilly, AstraZeneca, GlaxoSmithKlein(GSK), Kezar Life Sciences, Bristol-Myers Squibb(BMS); M. Clowse, Exagen; S. Ballou, None; L. Ding, None; B. Welch, None; J. Springer, None; A. Shaw, None; L. Keyes-Elstein, None; K. Steinmiller, None; A. Mickey, None; C. Aranow, None; B. Diamond, None.

