Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0372–0402) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster I
0397: Aberrant Global DNA Methylation in Peripheral Blood Cell Subpopulations of Patients with Axial Spondyloarthritis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- ET
Eric Toussirot, MD
university hospital of BESANCON
besancon, France
Abstract Poster Presenter(s)
ERIC TOUSSIROT1, Sebastien Pasquereau2, Charline Vauchy3, Daniel Wendling4, Jean Charles Balblanc5, caroline Laheurte6, Marc Puyraveau7 and Georges Herbein2, 1CHU de Besançon, Besançon, France, 2EA EPILAB, Besancon, Besançon, France, 3INSERM CIC-1431, Besancon, Besançon, France, 4CHU, University Teaching Hospital, Besançon, France, 5Rhumatologie, Hopital Nord Franche Comté, Trevenans, France, 6EFS Bourgogne Franche Comté, Biomonotoring Plateform, Besançon, France, 7INSERM CIC-1431 CHU, Besançon, France
Background/Purpose: Axial spondyloarthritis (axSpA) corresponds to a group of chronic inflammatory diseases mainly affecting the axial skeleton. TNFa and IL-17A have been identified as key inflammatory mediators driving the inflammatory process of axSpA. Epigenetics refers to different mechanisms that alter gene expression without involving changes in DNA sequence. DNA methylation is an important epigenetic mechanism, playing a role in gene expression regulation. It is recognized that aberrant DNA methylation can result in immune cell autoreactivity. In this study, we aimed to evaluate the global DNA methylation of patients with axSpA.
Methods: Case-control study (NCT03092583). Patients with r-axSpA or non radiographic (nr) axSpA (ASAS criteria) and healthy controls (HC) were evaluated. All the patients were biologic naïve and under NSAIDs. Disease activity was evaluated by BASDAI and ASDAS. CD4+T cells and CD14+ monocytes were isolated from peripheral blood and then DNA was extracted. Global DNA methylation (5-mC) was determined using MethylAmp global DNA methylation quantification kit (Epigentek) using 150 ng of total DNA.
Results: 104 patients with axSpA including 59 with r-axSpA (45 M; age [mean ± SD]: 47.1 ± 15 y; disease duration: 15.2 ± 13 y; B27: 86.4%) and 45 nr-axSpA (21 M, age: 39.6 ± 13.2; disease duration: 7 ± 7.8; B27: 65.1%) and 79 healthy controls (HC) (51 M; age: 43.4 ± 12.2 y) were evaluated. Patients had active disease (BASDAI and ASDAS in r-axSpA and nr- axSpA: 5.1 ± 1.8 and 3.04 ± 1.1; 5.04 ± 1.1 and 2.8 ± 1.0, respectively). In CD4+ T lymphocytes, global DNA methylation was higher in the whole SpA group compared to HC (1.45 ± 3.6 vs 0.44 ± 0.9 of 5-mC (p= 0.0092). Similarly, DNA methylation was higher in monocytes from patients with ax-SpA compared to HC (1.94 ± 2.3 vs 0.62 ± 0.9 of 5-mC)(p= 0.0004). When analysing the results between ax-SpA subgroups, DNA methylation remains higher in both CD4 T lymphocytes and monocytes of each patient subgroup compared to HC (p< 0.05 for all comparisons) (Figure). The levels of DNA methylation did not correlate with laboratory (ESR, CRP) or clinical (BASDAI) measures of disease activity, excepting ASDAS which was weakly correlated with DNA methylation in CD4+ T lymphocytes from the whole group of axSpA (r = 0.18, p = 0.08). Previous smoker patients had higher DNA methylation in their monocytes compared to current or never smoker patients (p = 0.06). In a limited number of patients (N = 15) who started a TNFi, DNA methylation decreased in both CD4 T lymphocytes and monocytes after 3 months of treatment.
Conclusion: a global DNA hypermethylation was observed in patients with axSpA, both in T CD4 lymphocytes and monocytes. These modifications involved both the radiographic and non radiographic forms. Collectively, these changes in DNA methylation could alter recruitment of methyl binding proteins (MBP) that regulate chromatin structure and/or impair binding of transcription factors, resulting in down regulation of gene expression relevant to the pathogenesis of axSpA. We currently evaluated the level of expression of DNA methyltransferase (DNMT) and MBP proteins and specific DNA methylation status of the promoters of gene involved in inflammation such as TNFa.
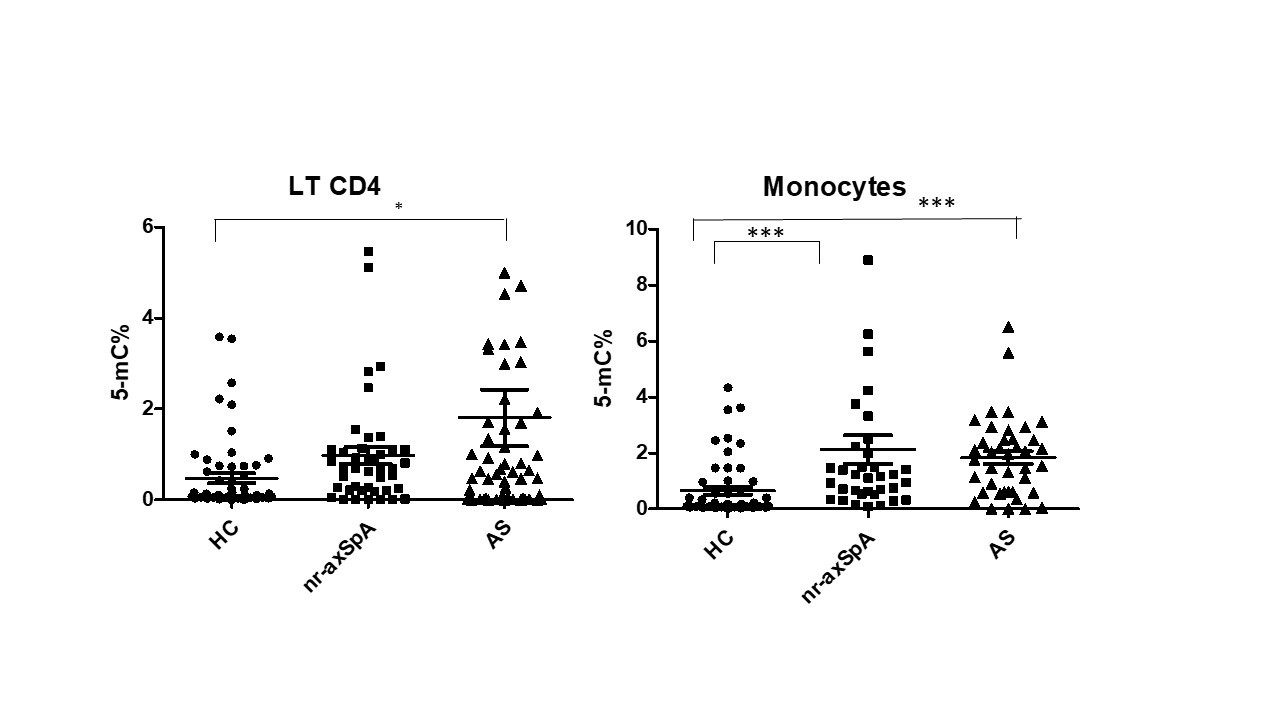 global DNA methylation of CD4+ T lymphocytes and monocytes from patients with ankylosing spondylitis (AS or radiographic axial spondyloarthritis) and non radiographic axial spondyloarthritis (nr-axSpA) and healthy controls (HC) (* p < 0.05; *** p < 0.001)
global DNA methylation of CD4+ T lymphocytes and monocytes from patients with ankylosing spondylitis (AS or radiographic axial spondyloarthritis) and non radiographic axial spondyloarthritis (nr-axSpA) and healthy controls (HC) (* p < 0.05; *** p < 0.001)
Disclosures: E. TOUSSIROT, None; S. Pasquereau, None; C. Vauchy, None; D. Wendling, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Pfizer, Roche, Amgen, Nordic Pharma, UCB, Novartis, Janssen, Eli Lilly, Sandoz, Galapados, Grunenthal; J. Balblanc, None; c. Laheurte, None; M. Puyraveau, None; G. Herbein, None.
Background/Purpose: Axial spondyloarthritis (axSpA) corresponds to a group of chronic inflammatory diseases mainly affecting the axial skeleton. TNFa and IL-17A have been identified as key inflammatory mediators driving the inflammatory process of axSpA. Epigenetics refers to different mechanisms that alter gene expression without involving changes in DNA sequence. DNA methylation is an important epigenetic mechanism, playing a role in gene expression regulation. It is recognized that aberrant DNA methylation can result in immune cell autoreactivity. In this study, we aimed to evaluate the global DNA methylation of patients with axSpA.
Methods: Case-control study (NCT03092583). Patients with r-axSpA or non radiographic (nr) axSpA (ASAS criteria) and healthy controls (HC) were evaluated. All the patients were biologic naïve and under NSAIDs. Disease activity was evaluated by BASDAI and ASDAS. CD4+T cells and CD14+ monocytes were isolated from peripheral blood and then DNA was extracted. Global DNA methylation (5-mC) was determined using MethylAmp global DNA methylation quantification kit (Epigentek) using 150 ng of total DNA.
Results: 104 patients with axSpA including 59 with r-axSpA (45 M; age [mean ± SD]: 47.1 ± 15 y; disease duration: 15.2 ± 13 y; B27: 86.4%) and 45 nr-axSpA (21 M, age: 39.6 ± 13.2; disease duration: 7 ± 7.8; B27: 65.1%) and 79 healthy controls (HC) (51 M; age: 43.4 ± 12.2 y) were evaluated. Patients had active disease (BASDAI and ASDAS in r-axSpA and nr- axSpA: 5.1 ± 1.8 and 3.04 ± 1.1; 5.04 ± 1.1 and 2.8 ± 1.0, respectively). In CD4+ T lymphocytes, global DNA methylation was higher in the whole SpA group compared to HC (1.45 ± 3.6 vs 0.44 ± 0.9 of 5-mC (p= 0.0092). Similarly, DNA methylation was higher in monocytes from patients with ax-SpA compared to HC (1.94 ± 2.3 vs 0.62 ± 0.9 of 5-mC)(p= 0.0004). When analysing the results between ax-SpA subgroups, DNA methylation remains higher in both CD4 T lymphocytes and monocytes of each patient subgroup compared to HC (p< 0.05 for all comparisons) (Figure). The levels of DNA methylation did not correlate with laboratory (ESR, CRP) or clinical (BASDAI) measures of disease activity, excepting ASDAS which was weakly correlated with DNA methylation in CD4+ T lymphocytes from the whole group of axSpA (r = 0.18, p = 0.08). Previous smoker patients had higher DNA methylation in their monocytes compared to current or never smoker patients (p = 0.06). In a limited number of patients (N = 15) who started a TNFi, DNA methylation decreased in both CD4 T lymphocytes and monocytes after 3 months of treatment.
Conclusion: a global DNA hypermethylation was observed in patients with axSpA, both in T CD4 lymphocytes and monocytes. These modifications involved both the radiographic and non radiographic forms. Collectively, these changes in DNA methylation could alter recruitment of methyl binding proteins (MBP) that regulate chromatin structure and/or impair binding of transcription factors, resulting in down regulation of gene expression relevant to the pathogenesis of axSpA. We currently evaluated the level of expression of DNA methyltransferase (DNMT) and MBP proteins and specific DNA methylation status of the promoters of gene involved in inflammation such as TNFa.
 global DNA methylation of CD4+ T lymphocytes and monocytes from patients with ankylosing spondylitis (AS or radiographic axial spondyloarthritis) and non radiographic axial spondyloarthritis (nr-axSpA) and healthy controls (HC) (* p < 0.05; *** p < 0.001)
global DNA methylation of CD4+ T lymphocytes and monocytes from patients with ankylosing spondylitis (AS or radiographic axial spondyloarthritis) and non radiographic axial spondyloarthritis (nr-axSpA) and healthy controls (HC) (* p < 0.05; *** p < 0.001)Disclosures: E. TOUSSIROT, None; S. Pasquereau, None; C. Vauchy, None; D. Wendling, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Pfizer, Roche, Amgen, Nordic Pharma, UCB, Novartis, Janssen, Eli Lilly, Sandoz, Galapados, Grunenthal; J. Balblanc, None; c. Laheurte, None; M. Puyraveau, None; G. Herbein, None.

