Back
Poster Session C
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: (1360–1386) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
1374: Real-World Effectiveness and Steroid Sparing Effect of Belimumab in Pediatric Lupus: A Single Center Retrospective Study
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Jordan Roberts, MD, MPH
Seattle Children's Hospital
Seattle, WA, United States
Abstract Poster Presenter(s)
Jordan Roberts1, Mindy Lo1, Rebecca Sadun2, Emily Smitherman3, Scott Wenderfer4 and Mary Beth F. Son1, 1Division of Immunology, Boston Children's Hospital, Boston, MA, 2Duke University, Durham, NC, 3University of Alabama at Birmingham, Birmingham, AL, 4British Columbia Children's Hospital, Vancouver, BC, Canada
Background/Purpose: Belimumab is the only targeted therapy approved for pediatric systemic lupus erythematosus (pSLE), although use remains limited in children. While clinical trials and studies of real-world effectiveness in adults showed improved disease control and decreased oral glucocorticoid use, effectiveness has not been studied in children outside of the trial setting. We aimed to characterize patterns of belimumab use at a single large pediatric lupus center and determine whether addition of belimumab led to lower oral glucocorticoid doses and disease activity scores in the year following initiation.
Methods: We included children and young adults with pSLE treated at Boston Children's Hospital who received ≥ 1 dose of belimumab. Patients were identified from the electronic health record via J-code or prescription for belimumab, and pSLE diagnosis was confirmed via chart review. Summary statistics were performed to describe demographic and disease features, indications for belimumab, and combination therapy. Repeated measures one-way ANOVA was used to compare SLEDAI-2K scores and prednisone-equivalent daily oral glucocorticoid doses at baseline, 6 and 12 months after belimumab initiation for those who continued on therapy.
Results: We identified 21 patients with pSLE who received ≥ 1 dose of belimumab. All started on intravenous; 4 transitioned to subcutaneous. Patient characteristics and the most common indications for belimumab are shown in Table 1. Median age at pSLE diagnosis was 12 years. Median disease duration at belimumab initiation was 31 months [IQR 21-79]. At time of belimumab initiation, 100% of patients were taking an antimalarial, 81% were on oral glucocorticoids, and 91% were on at least one conventional DMARD (Table 2). Sixty-two percent had received rituximab and 48% had received cyclophosphamide prior to belimumab; 67% had received at least one. Thirteen patients (62%) continued belimumab for ≥ 6 months, and 11 (52%) for ≥12 months; median follow up time on belimumab was 11 months. Top reasons for discontinuation were lack of clinical improvement or transition to other therapy (29%), difficulty coming to infusion appointments (19%) and non-adherence (19%). One patient had infection (appendicitis) prompting discontinuation. Steroid and SLEDAI-2K trajectories for those with ≥ 12 months on belimumab are shown in Figure 1. Median [IQR] SLEDAI-2K scores at baseline, 6 month and 12 months were 9 [6-11], 7 [4-12] and 6 [6-8.5] respectively, p=0.548. Median [IQR] oral prednisone daily doses in mg at baseline, 6 month and 12 months were 12.5 [5-20], 8.9 [4.375-10], and 6.9 [5-10], p=0.037.
Conclusion: In our single center cohort of pediatric patients with long-standing, refractory lupus and moderate disease activity who continued belimumab therapy for ≥ 12 months, daily oral glucorticoid doses were significantly lower 6 and 12 months after belimumab initiation compared to baseline. SLEDAI-2K scores were numerically reduced at 6 and 12 months, but this was not statistically significant in our small cohort. Use in patients with active nephritis was uncommon. Further research is needed in large, multicenter cohorts to determine the effectiveness of belimumab in children and develop guidelines for optimal use.
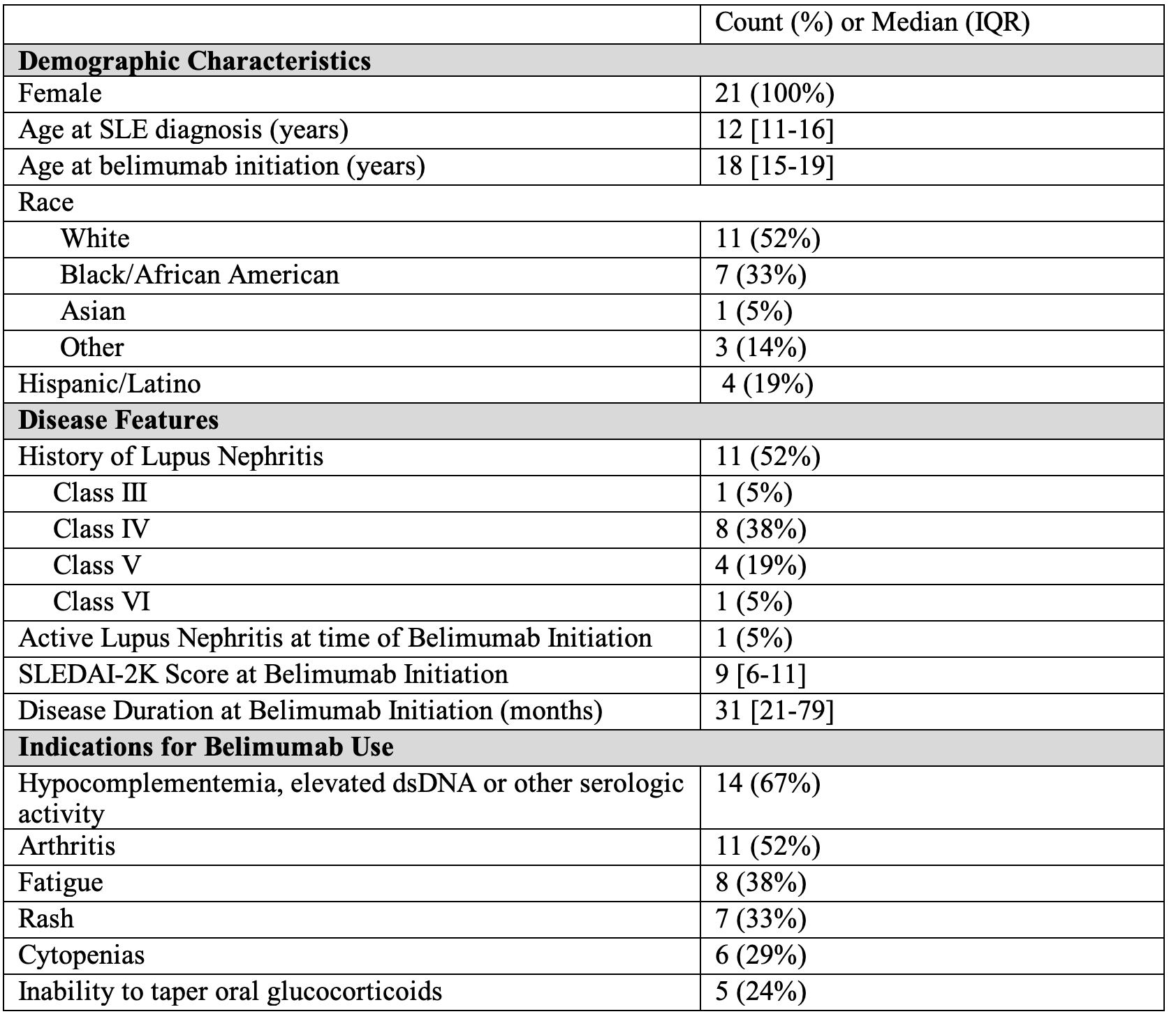 Table 1. Patient Characteristics at Belimumab Initiation
Table 1. Patient Characteristics at Belimumab Initiation
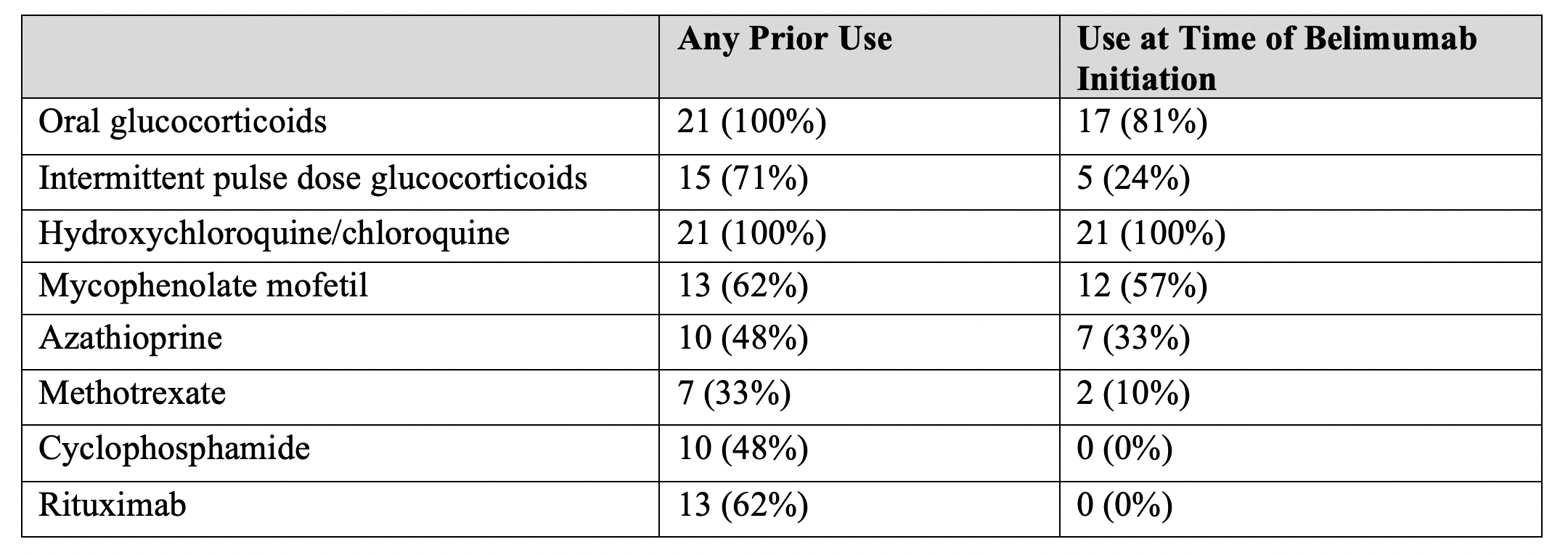 Table 2. Co-Administered and Previously Used Medications for pSLE
Table 2. Co-Administered and Previously Used Medications for pSLE
.jpg) Figure 1. SLEDAI-2K Scores and Oral Prednisone Doses Over First Year of Treatment
Figure 1. SLEDAI-2K Scores and Oral Prednisone Doses Over First Year of Treatment
Disclosures: J. Roberts, None; M. Lo, GlaxoSmithKlein(GSK); R. Sadun, None; E. Smitherman, None; S. Wenderfer, Bristol-Myers Squibb(BMS), Alnylam; M. Son, None.
Background/Purpose: Belimumab is the only targeted therapy approved for pediatric systemic lupus erythematosus (pSLE), although use remains limited in children. While clinical trials and studies of real-world effectiveness in adults showed improved disease control and decreased oral glucocorticoid use, effectiveness has not been studied in children outside of the trial setting. We aimed to characterize patterns of belimumab use at a single large pediatric lupus center and determine whether addition of belimumab led to lower oral glucocorticoid doses and disease activity scores in the year following initiation.
Methods: We included children and young adults with pSLE treated at Boston Children's Hospital who received ≥ 1 dose of belimumab. Patients were identified from the electronic health record via J-code or prescription for belimumab, and pSLE diagnosis was confirmed via chart review. Summary statistics were performed to describe demographic and disease features, indications for belimumab, and combination therapy. Repeated measures one-way ANOVA was used to compare SLEDAI-2K scores and prednisone-equivalent daily oral glucocorticoid doses at baseline, 6 and 12 months after belimumab initiation for those who continued on therapy.
Results: We identified 21 patients with pSLE who received ≥ 1 dose of belimumab. All started on intravenous; 4 transitioned to subcutaneous. Patient characteristics and the most common indications for belimumab are shown in Table 1. Median age at pSLE diagnosis was 12 years. Median disease duration at belimumab initiation was 31 months [IQR 21-79]. At time of belimumab initiation, 100% of patients were taking an antimalarial, 81% were on oral glucocorticoids, and 91% were on at least one conventional DMARD (Table 2). Sixty-two percent had received rituximab and 48% had received cyclophosphamide prior to belimumab; 67% had received at least one. Thirteen patients (62%) continued belimumab for ≥ 6 months, and 11 (52%) for ≥12 months; median follow up time on belimumab was 11 months. Top reasons for discontinuation were lack of clinical improvement or transition to other therapy (29%), difficulty coming to infusion appointments (19%) and non-adherence (19%). One patient had infection (appendicitis) prompting discontinuation. Steroid and SLEDAI-2K trajectories for those with ≥ 12 months on belimumab are shown in Figure 1. Median [IQR] SLEDAI-2K scores at baseline, 6 month and 12 months were 9 [6-11], 7 [4-12] and 6 [6-8.5] respectively, p=0.548. Median [IQR] oral prednisone daily doses in mg at baseline, 6 month and 12 months were 12.5 [5-20], 8.9 [4.375-10], and 6.9 [5-10], p=0.037.
Conclusion: In our single center cohort of pediatric patients with long-standing, refractory lupus and moderate disease activity who continued belimumab therapy for ≥ 12 months, daily oral glucorticoid doses were significantly lower 6 and 12 months after belimumab initiation compared to baseline. SLEDAI-2K scores were numerically reduced at 6 and 12 months, but this was not statistically significant in our small cohort. Use in patients with active nephritis was uncommon. Further research is needed in large, multicenter cohorts to determine the effectiveness of belimumab in children and develop guidelines for optimal use.
 Table 1. Patient Characteristics at Belimumab Initiation
Table 1. Patient Characteristics at Belimumab Initiation Table 2. Co-Administered and Previously Used Medications for pSLE
Table 2. Co-Administered and Previously Used Medications for pSLE.jpg) Figure 1. SLEDAI-2K Scores and Oral Prednisone Doses Over First Year of Treatment
Figure 1. SLEDAI-2K Scores and Oral Prednisone Doses Over First Year of TreatmentDisclosures: J. Roberts, None; M. Lo, GlaxoSmithKlein(GSK); R. Sadun, None; E. Smitherman, None; S. Wenderfer, Bristol-Myers Squibb(BMS), Alnylam; M. Son, None.

