Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1417–1439) RA – Treatment Poster III
1434: Retrospective Analysis of Tocilizumab-induced Neutropenia in Patients with Rheumatoid Arthritis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- YK
YoungEun Kim, MD
Department of Rheumatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
Seoul, South Korea
Abstract Poster Presenter(s)
YoungEun Kim1, Soo Min Ahn1, Ji Seon Oh2, Yong Gil Kim1, Chang Keun Lee1, Bin Yoo1 and Seokchan Hong1, 1Department of Rheumatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea, Seoul, Republic of Korea, 2Department of Information Medicine, Big Data Research Center, Asan Medical Center, Seoul, Republic of Korea
Background/Purpose: Neutropenia has been reported as one of the most frequent adverse events of tocilizumab. We conducted a retrospective cohort study to determine the risks and clinical significance of tocilizumab-induced neutropenia, in real-world settings, for patients with rheumatoid arthritis (RA).
Methods: We collected the medical records of patients with RA who were treated with tocilizumab, between January 2013 and December 2021, at a tertiary referral hospital in Seoul, South Korea. Data regarding complete blood cell counts were collected. Neutropenia was graded following the Common Terminology Criteria for Adverse Events guidelines as follows: grade 0, normal; grade 1, < lower limit of normal (LLN) to 1.5 × 109/L; grade 2, < 1.5 to 1.0 × 109/L; grade 3, < 1.0 to 0.5 × 109/L; and grade 4, < 0.5 × 109/L. Infectious complications were confirmed by clinical diagnosis and treated with antibiotics.
Results: A total of 277 patients with RA, who received tocilizumab treatment, were included in our study. Of the total patients, tocilizumab was administered as subcutaneous and intravenous injections in 152 (54.8%) and 125 (45.1%) patients, respectively, during 782 patient-years (PY). During the follow-up period, 22 (7%) patients experienced grade 3/4 neutropenia. None of the patients discontinued tocilizumab due to neutropenia, while the dosage of conventional synthetic DMARD (csDMARD) was either reduced or discontinued for 8 patients. There was no significant difference in the time taken for recovery from neutropenia, between the csDMARD discontinuation group (median, 1.6 mo [IQR: 1.0–2.0]) and the csDMARD continuation group (median, 2.6 mo [IQR: 1.0–5.3]) (P=0.58). A previous history of neutropenia during treatment with csDMARD could contribute to a higher risk for onset of grade 3/4 neutropenia (HR: 3.120; 95% CI: 1.189-8.189; P=0.021). In the enrolled population, infections occurred during tocilizumab treatment primarily included pulmonary infections at 10.31/100 PY (pneumonia at 4.86/100 PY, other acute lower respiratory infections at 5.45/100 PY). Age over 60 years and the presence of extra-articular manifestations were significantly associated with a higher risk of infections (Age, HR: 2.133; 95% CI: 1.118–4.071, P=0.022; extra-articular manifestations, HR: 14.566, 95% CI: 6.317-33.584, P< 0.0001). However, grade 3/4 neutropenia induced by tocilizumab administration was not associated with increased infection risk (HR: 0.721; 95% CI: 0.105-2.117; P=0.471).
Conclusion: Approximately 7% of patients with RA treated with tocilizumab developed grade 3/4 neutropenia. A previous history of neutropenia due to csDMARD therapy was an important predictor for the onset of tocilizumab-induced neutropenia. Age and extra-articular manifestations, but not neutropenia, were associated with infection, indicating that tocilizumab-induced neutropenia was not clinically significant in the incidence of infection in patients with RA.
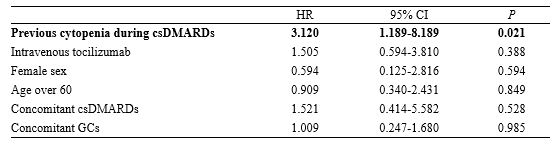 Risk factors for the development of grade 3/4 neutropenia in patients with tocilizumab
Risk factors for the development of grade 3/4 neutropenia in patients with tocilizumab
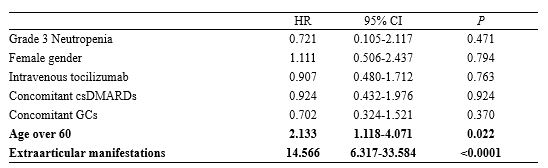 Multivariable analysis of the risk factors for infection during tocilizumab treatment
Multivariable analysis of the risk factors for infection during tocilizumab treatment
Disclosures: Y. Kim, None; S. Ahn, None; J. Oh, None; Y. Kim, None; C. Lee, None; B. Yoo, None; S. Hong, None.
Background/Purpose: Neutropenia has been reported as one of the most frequent adverse events of tocilizumab. We conducted a retrospective cohort study to determine the risks and clinical significance of tocilizumab-induced neutropenia, in real-world settings, for patients with rheumatoid arthritis (RA).
Methods: We collected the medical records of patients with RA who were treated with tocilizumab, between January 2013 and December 2021, at a tertiary referral hospital in Seoul, South Korea. Data regarding complete blood cell counts were collected. Neutropenia was graded following the Common Terminology Criteria for Adverse Events guidelines as follows: grade 0, normal; grade 1, < lower limit of normal (LLN) to 1.5 × 109/L; grade 2, < 1.5 to 1.0 × 109/L; grade 3, < 1.0 to 0.5 × 109/L; and grade 4, < 0.5 × 109/L. Infectious complications were confirmed by clinical diagnosis and treated with antibiotics.
Results: A total of 277 patients with RA, who received tocilizumab treatment, were included in our study. Of the total patients, tocilizumab was administered as subcutaneous and intravenous injections in 152 (54.8%) and 125 (45.1%) patients, respectively, during 782 patient-years (PY). During the follow-up period, 22 (7%) patients experienced grade 3/4 neutropenia. None of the patients discontinued tocilizumab due to neutropenia, while the dosage of conventional synthetic DMARD (csDMARD) was either reduced or discontinued for 8 patients. There was no significant difference in the time taken for recovery from neutropenia, between the csDMARD discontinuation group (median, 1.6 mo [IQR: 1.0–2.0]) and the csDMARD continuation group (median, 2.6 mo [IQR: 1.0–5.3]) (P=0.58). A previous history of neutropenia during treatment with csDMARD could contribute to a higher risk for onset of grade 3/4 neutropenia (HR: 3.120; 95% CI: 1.189-8.189; P=0.021). In the enrolled population, infections occurred during tocilizumab treatment primarily included pulmonary infections at 10.31/100 PY (pneumonia at 4.86/100 PY, other acute lower respiratory infections at 5.45/100 PY). Age over 60 years and the presence of extra-articular manifestations were significantly associated with a higher risk of infections (Age, HR: 2.133; 95% CI: 1.118–4.071, P=0.022; extra-articular manifestations, HR: 14.566, 95% CI: 6.317-33.584, P< 0.0001). However, grade 3/4 neutropenia induced by tocilizumab administration was not associated with increased infection risk (HR: 0.721; 95% CI: 0.105-2.117; P=0.471).
Conclusion: Approximately 7% of patients with RA treated with tocilizumab developed grade 3/4 neutropenia. A previous history of neutropenia due to csDMARD therapy was an important predictor for the onset of tocilizumab-induced neutropenia. Age and extra-articular manifestations, but not neutropenia, were associated with infection, indicating that tocilizumab-induced neutropenia was not clinically significant in the incidence of infection in patients with RA.
 Risk factors for the development of grade 3/4 neutropenia in patients with tocilizumab
Risk factors for the development of grade 3/4 neutropenia in patients with tocilizumab Multivariable analysis of the risk factors for infection during tocilizumab treatment
Multivariable analysis of the risk factors for infection during tocilizumab treatmentDisclosures: Y. Kim, None; S. Ahn, None; J. Oh, None; Y. Kim, None; C. Lee, None; B. Yoo, None; S. Hong, None.

