Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1524: Risk Factors for Lung Function Decline in Systemic Sclerosis Interstitial Lung Disease in a Large Single-Center Cohort
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AR
Ahmad Ramahi, MD, MPH
University of Michigan
Roswell, NM, United States
Abstract Poster Presenter(s)
Ahmad Ramahi1, Alain Lescoat2, David Roofeh1, Vivek Nagaraja1, Rajaie Namas3, Suiyuan Huang1, John Varga1, David O’Dwyer1, Bonnie Wang1, Kevin Flaherty1, Ella Kazerooni1 and Dinesh Khanna4, 1University of Michigan, Ann Arbor, MI, 2CHU Rennes - University Rennes 1, Rennes, France, 3Medical Subspecialties Institute, Division of Rheumatology at Cleveland Clinic, Abu Dhabi, United Arab Emirates, 4Division of Rheumatology, Department of Internal Medicine, Scleroderma Program, University of Michigan, Ann Arbor, MI
Background/Purpose: Systemic sclerosis-associated interstitial lung disease (SSc-ILD) is the leading cause of scleroderma-related mortality. This work identifies factors associated with SSc-ILD decline on pulmonary function testing (PFT).
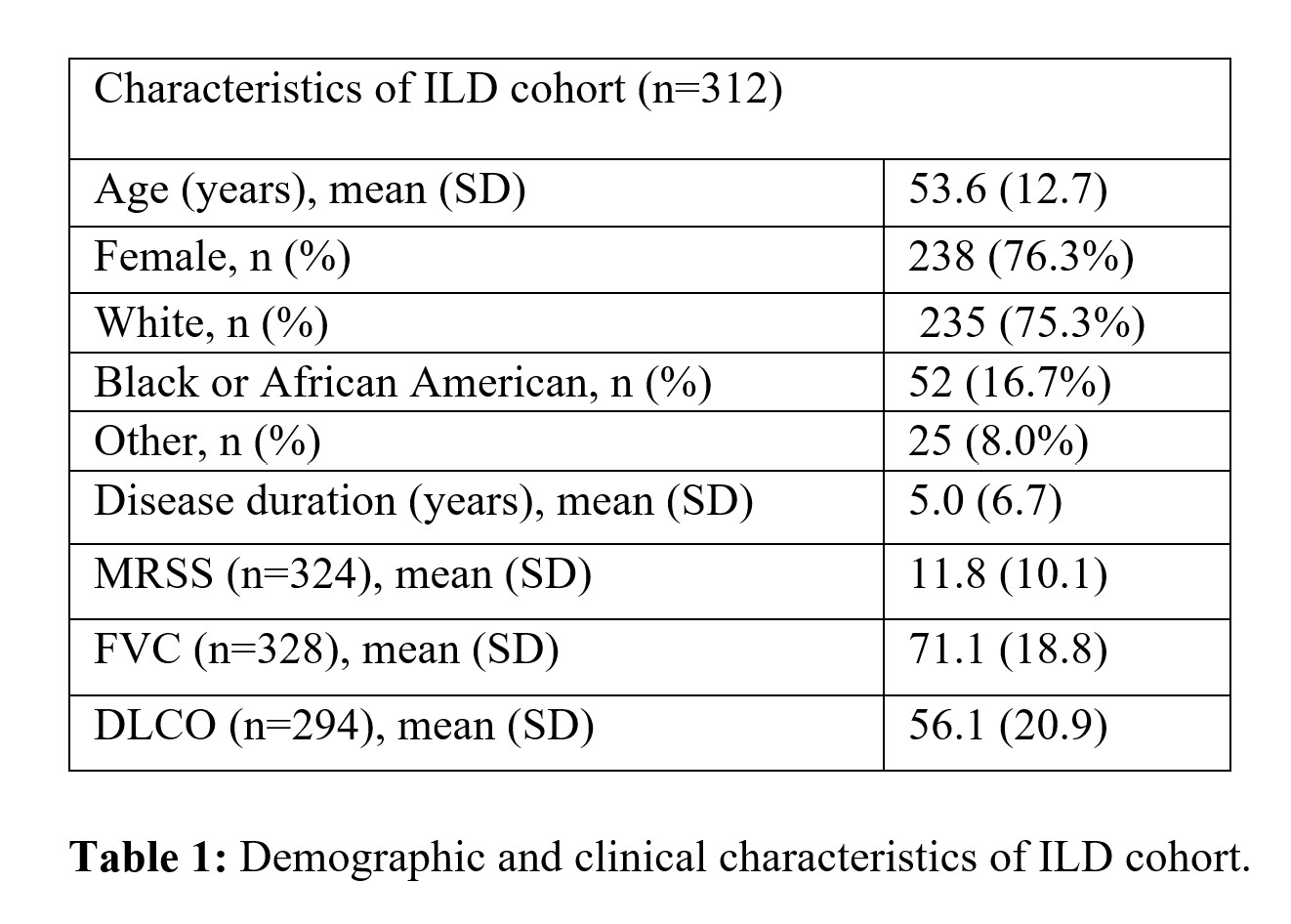
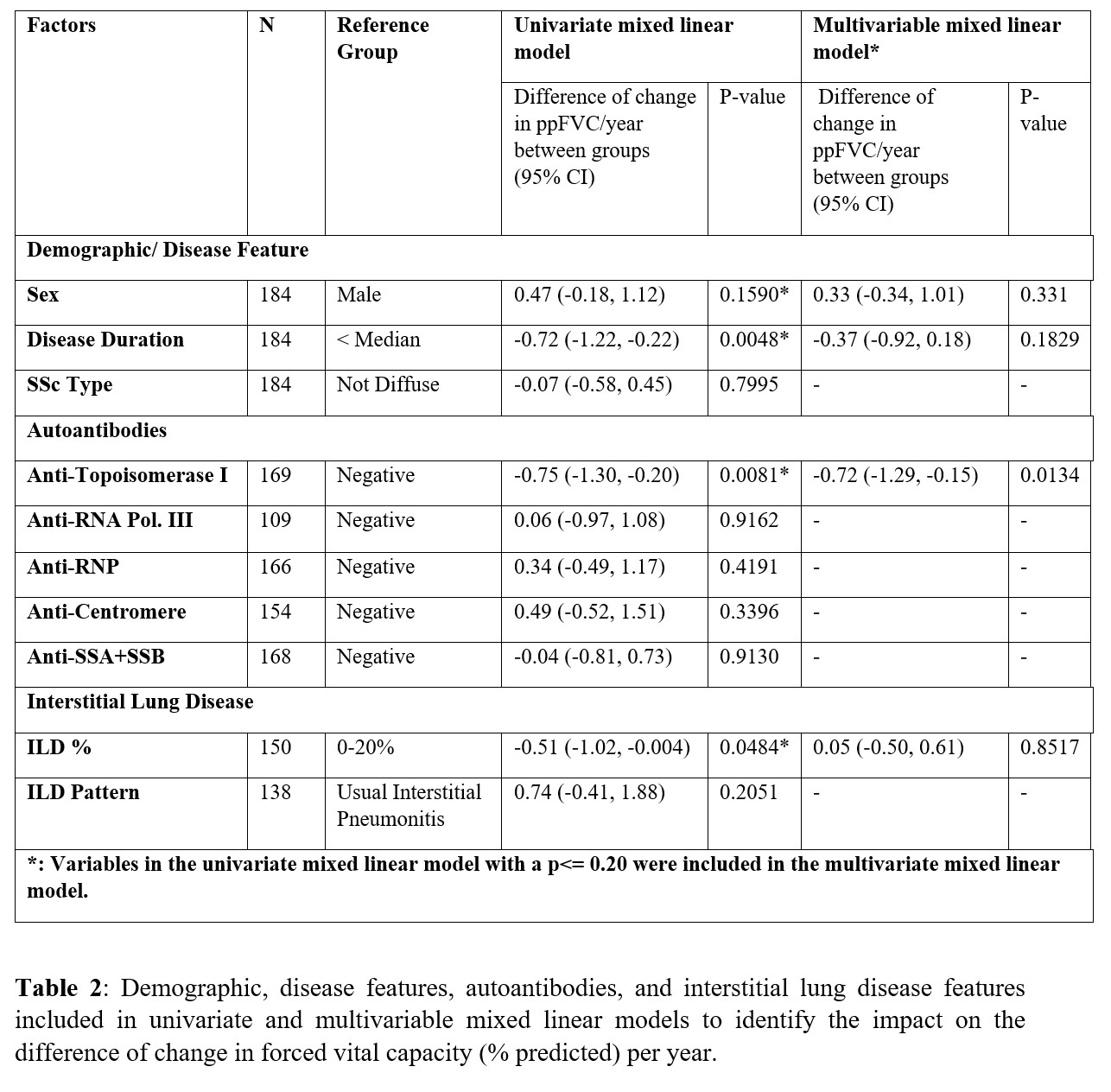
Methods: This single-center cohort identified 312 patients with ILD as determined by high-resolution chest from a total of 484 patients with systemic sclerosis (Table 1). 184 patients (59% of 312) completed baseline and serial PFTs (with at least two follow-up PFTs) and were included in this analysis (Table 2). Mixed linear models were fit to assess the decline in the percent predicted forced vital capacity (ppFVC) over time. Demographics, disease factors, autoantibodies, and ILD features were included in the univariate mixed linear model; those achieving a p-value < 0.20 were included in the multivariable mixed linear model. Patients were followed longitudinally, with survival as an endpoint identified using the National Death Registry Index, reviewing death certificates, and hospital records.
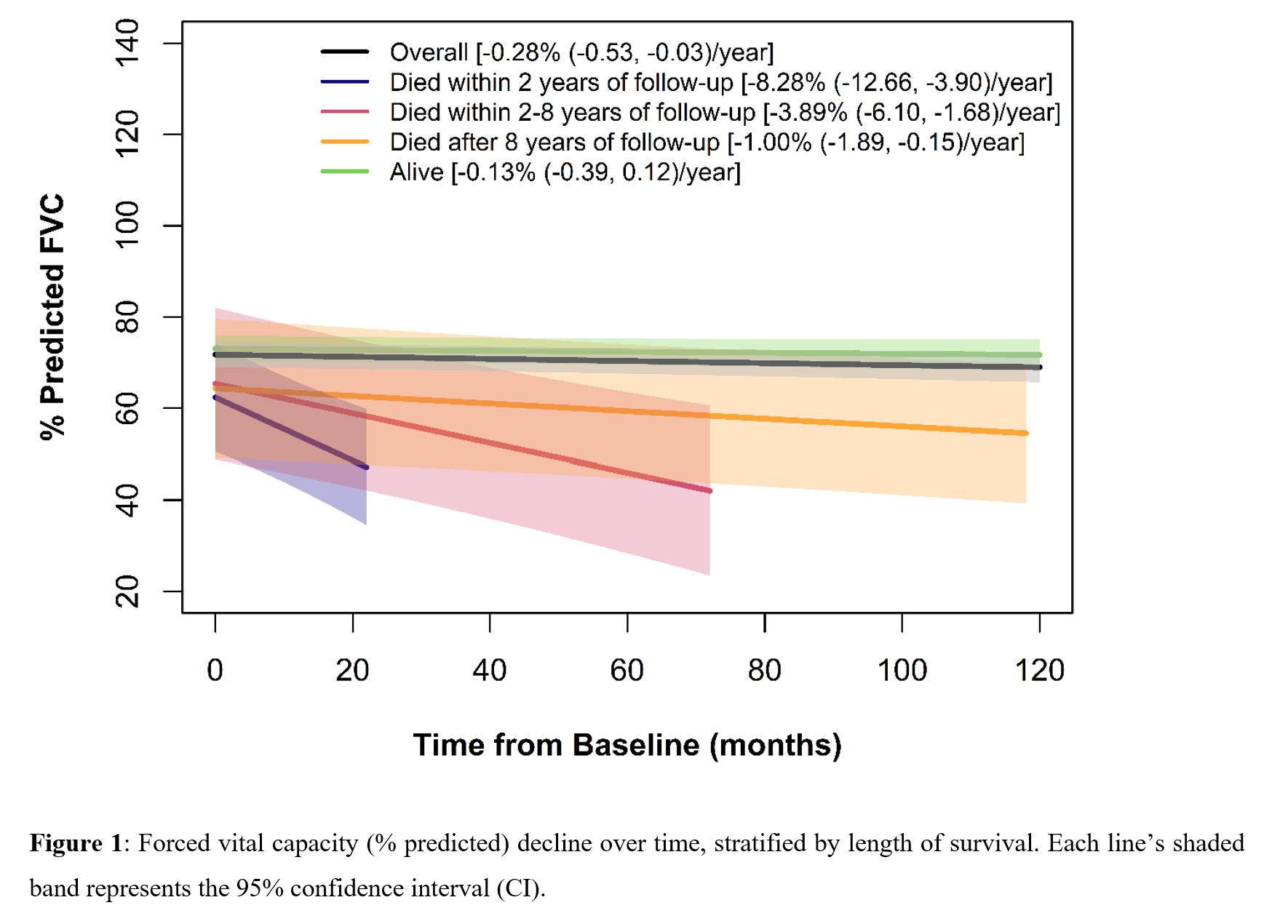
Results: The 184 patients were an average of 53.2 [12.1] years old; the median [IQR] disease duration from the first non-Raynaud's phenomenon symptom was 1.8 [0.7, 4.8] years. SSc subtype was diffuse in 55.4% (n=102), limited in 32.6% (n=60), overlap syndrome in 8.2% (n=15), and SSc sine scleroderma in 3.87% (n=7). Serologies were positive for anti-topoisomerase I (ATA), anti-centromere and anti-RNA polymerase III in 31.4% (n=53/169), 10.4% (16/154) and 22.9% (25/109) respectively. Mean ppFVC was 70.8 (18.9) and ppDLco 57.2 (20.8). Whole lung involvement (WLI%) of >20% on visual read was found in 49.3% of subjects (74/150 (49.3%)) where quantification was available. Over a median of 4.9 (2.4, 6.8) years, 21 patients (11.4%) died. The ppFVC declined a mean of 0.28/year in the overall group (Figure 1). The primary cause of death was ILD (6/21, 28.6%); those who died in the first 2 years most often died from progressive ILD (4/6, 67%). Table 2 reports factors significantly associated with decline in ppFVC (columns 4 and 5) on univariate analyses, including longer disease duration (ref. < median, P=0.0048), ATA positivity (ref. negative, P=0.0081), and WLI >20% (ref. 0-20%, P=0.0484). In multivariate analysis (columns 6 and 7) the only statistically significant variable associated with decline in ppFVC/ year was ATA positivity.
Conclusion: In a large single center cohort of SSc-ILD, ATA positivity is a risk factor for developing progressive SSc-ILD, consistent with other SSc-ILD cohorts. Stratifying patients by survival demonstrates that lung function declines dramatically in those who died within 2 years, whose main cause of death was progressive ILD. These data support the growing need to identify risk factors for disease severity and risk for progression and to target intervention in patients most likely to develop progressive SSc-ILD 1,2.
References
1. Roofeh D, Lin CJF, Goldin JG, et al. Tocilizumab Prevents Progression of Early Systemic Sclerosis Associated Interstitial Lung Disease. Arthritis Rheum. 2021:Online Ahead of Print.
2. Roofeh D, Lescoat A, Khanna D. Treatment for systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol. 2021;Publish Ah.
Disclosures: A. Ramahi, None; A. Lescoat, None; D. Roofeh, Boehringer-Ingelheim; V. Nagaraja, None; R. Namas, None; S. Huang, None; J. Varga, Boehringer-Ingelheim; D. O’Dwyer, None; B. Wang, None; K. Flaherty, Boehringer-Ingelheim, Genentech, AstraZeneca, fibrogen, Bellerophon, Respivant, Blade Therapeutics, Blade Therapeutics, Shionogi, DevPro biopharma, Pure Health, Horizon, Sun pharmaceuticals, Pliant, United Therapeutics, Arrowhead, Lupin, Polarean, Pure Tech, Trevi Pharmaceuticals, CSL Behring, Daewong pharmaceuticals, Dispersol, Immunmet, NeRRe Therapeutics; E. Kazerooni, None; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences.
Background/Purpose: Systemic sclerosis-associated interstitial lung disease (SSc-ILD) is the leading cause of scleroderma-related mortality. This work identifies factors associated with SSc-ILD decline on pulmonary function testing (PFT).
Methods: This single-center cohort identified 312 patients with ILD as determined by high-resolution chest from a total of 484 patients with systemic sclerosis (Table 1). 184 patients (59% of 312) completed baseline and serial PFTs (with at least two follow-up PFTs) and were included in this analysis (Table 2). Mixed linear models were fit to assess the decline in the percent predicted forced vital capacity (ppFVC) over time. Demographics, disease factors, autoantibodies, and ILD features were included in the univariate mixed linear model; those achieving a p-value < 0.20 were included in the multivariable mixed linear model. Patients were followed longitudinally, with survival as an endpoint identified using the National Death Registry Index, reviewing death certificates, and hospital records.
Results: The 184 patients were an average of 53.2 [12.1] years old; the median [IQR] disease duration from the first non-Raynaud's phenomenon symptom was 1.8 [0.7, 4.8] years. SSc subtype was diffuse in 55.4% (n=102), limited in 32.6% (n=60), overlap syndrome in 8.2% (n=15), and SSc sine scleroderma in 3.87% (n=7). Serologies were positive for anti-topoisomerase I (ATA), anti-centromere and anti-RNA polymerase III in 31.4% (n=53/169), 10.4% (16/154) and 22.9% (25/109) respectively. Mean ppFVC was 70.8 (18.9) and ppDLco 57.2 (20.8). Whole lung involvement (WLI%) of >20% on visual read was found in 49.3% of subjects (74/150 (49.3%)) where quantification was available. Over a median of 4.9 (2.4, 6.8) years, 21 patients (11.4%) died. The ppFVC declined a mean of 0.28/year in the overall group (Figure 1). The primary cause of death was ILD (6/21, 28.6%); those who died in the first 2 years most often died from progressive ILD (4/6, 67%). Table 2 reports factors significantly associated with decline in ppFVC (columns 4 and 5) on univariate analyses, including longer disease duration (ref. < median, P=0.0048), ATA positivity (ref. negative, P=0.0081), and WLI >20% (ref. 0-20%, P=0.0484). In multivariate analysis (columns 6 and 7) the only statistically significant variable associated with decline in ppFVC/ year was ATA positivity.
Conclusion: In a large single center cohort of SSc-ILD, ATA positivity is a risk factor for developing progressive SSc-ILD, consistent with other SSc-ILD cohorts. Stratifying patients by survival demonstrates that lung function declines dramatically in those who died within 2 years, whose main cause of death was progressive ILD. These data support the growing need to identify risk factors for disease severity and risk for progression and to target intervention in patients most likely to develop progressive SSc-ILD 1,2.
References
1. Roofeh D, Lin CJF, Goldin JG, et al. Tocilizumab Prevents Progression of Early Systemic Sclerosis Associated Interstitial Lung Disease. Arthritis Rheum. 2021:Online Ahead of Print.
2. Roofeh D, Lescoat A, Khanna D. Treatment for systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol. 2021;Publish Ah.



Disclosures: A. Ramahi, None; A. Lescoat, None; D. Roofeh, Boehringer-Ingelheim; V. Nagaraja, None; R. Namas, None; S. Huang, None; J. Varga, Boehringer-Ingelheim; D. O’Dwyer, None; B. Wang, None; K. Flaherty, Boehringer-Ingelheim, Genentech, AstraZeneca, fibrogen, Bellerophon, Respivant, Blade Therapeutics, Blade Therapeutics, Shionogi, DevPro biopharma, Pure Health, Horizon, Sun pharmaceuticals, Pliant, United Therapeutics, Arrowhead, Lupin, Polarean, Pure Tech, Trevi Pharmaceuticals, CSL Behring, Daewong pharmaceuticals, Dispersol, Immunmet, NeRRe Therapeutics; E. Kazerooni, None; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences.

