Back
Poster Session C
Systemic lupus erythematosus (SLE)
Session: (1440–1485) SLE – Diagnosis, Manifestations, and Outcomes Poster II: Manifestations
1442: Role of Platelet-bound Complement Activation Product (PC4d) in Predicting Risk of Future Thrombotic Events in Systemic Lupus Erythematosus
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
- AA
Anca Askanase, MD, MPH
Director
Columbia University Medical Center
New York, NY, United States
Abstract Poster Presenter(s)
Anca Askanase1, John Conklin2, Michelle Petri3, Vasileios Kyttaris4, Yevgeniya Gartshteyn5, Wei Tang1, Anja Kammesheidt6 and Roberta Alexander2, 1Columbia University Medical Center, New York, NY, 2Exagen, Inc., Vista, CA, 3Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 4Beth Israel Deaconess Medical Center, Boston, MA, 5Columbia University Medical Center, Glen Rock, NJ, 6self, Laguna Beach, CA
Background/Purpose: Platelet-bound complement activation products (PC4d) are associated with a history of thrombosis in systemic lupus erythematosus (SLE) (Gartshteyn al., 2021; Petri et al., 2017). The current study evaluated the role of PC4d in assessing the risk of future thrombosis.
Methods: PC4d (expressed at net mean fluorescence intensity [MFI]) was measured by flow cytometry from patients enrolled in the Lupus Cohorts at Columbia Univ (COL), Johns Hopkins Univ (JH) and Beth Israel Deaconess Medical Center (BI) between Apr-Sep 2017 (JH), Aug 2018-Jan 2020 (COL), and Oct 2018-Mar 2022 (BI). History of thrombotic vascular events was confirmed by medical record review. Data were analyzed by chi-square and logistic regression. Diagnostic odds ratio (DOR) was calculated. If a patient had 2 arterial or 2 venous events, only the closest in time to PC4d measurement was included in the analysis.
Results: A total of 419 SLE patients were enrolled. Main demographic and clinical characteristics are in Table 1. Seventy-four thrombotic events occurred in the 5 years pre- to 3 years post-PC4d measurement. Of these 74, 50% had PC4d≥10 MFI at enrollment while 72% of patients without thrombosis had PC4d< 10 (p< 0.001). 19 events occurred in 15 subjects in the 3 years after PC4d measurement: 8 cerebrovascular accidents, 3 deep vein thrombosis, 2 gastrointestinal infarctions, 2 myocardial infarctions, 1 pulmonary infarction, 1 arterial thrombosis and 2 venous thrombosis not specified. Median PC4d was higher in the 15 patients with than in the 404 patients without thrombosis (12.2 MFI, IQR: 4.7-19.6 vs. 4.9 MFI, IQR: 2.6-13.6, p=NS) (Figure 1). When the arterial events closest to PC4d measurements (8 of 14 total arterial events) were included, PC4d >13 MFI had sensitivity=62%, specificity=74%, DOR=4.8 (95%CI: 1.1, 20.3), p=0.034 in predicting future arterial thrombosis in patients with a previous arterial thrombosis. As expected, a previous arterial thrombosis was a strong predictor of a subsequent arterial event (p=0.0042). PC4d≥10 or ≥20 MFI did not reach statistical significance for future thrombosis neither in the entire population (p=0.055 and 0.51, respectively) nor in the subgroup younger than 65 years old (p = 0.99 for both). However, the negative predictive value of PC4d< 10 MFI was 98%, indicating that probability of not having a thrombosis within 3 years is 98% if PC4d< 10 MFI. Of the 285 patients with PC4d< 10 MFI, 105 (37%) were positive for at least 1 of 8 anti-phospholipid antibodies (aPL) (3 aCL, 3 anti-B2GP1, or 2 anti-PS/PT antibodies), and 22 (7.8%) were positive for 3 aPL, indicating that the probability of not having a thrombosis if PC4d< 10 MFI holds true for the population aPL positive and presumed at higher risk.
Conclusion: PC4d≥10 MFI is associated with thrombosis in SLE and predicts future arterial thrombosis (DOR=4.8), despite the small number of events post-PC4d. Interestingly, patients with PC4d < 10 MFI have a 98% probability of not experiencing a thrombotic event in the following 3 years. Taken together, these findings suggest that PC4d levels help evaluate the risk of thrombosis in SLE and can guide the decision to start low dose aspirin.
.gif) Table 1. Demographic characteristics of the patients in the 3 cohorts and overall.
Table 1. Demographic characteristics of the patients in the 3 cohorts and overall.
Data is reported as number (n) and percentages (%). Anti-phospholipid (aPL) positivity was based on positivity for any of the isotypes: anti-phosphatidylserine/prothrombin complex (PS/PT) IgG or IgM, anti-cardiolipin (aCL) IgG, IgM, or IgA, and anti-beta2-glycoprotein1 (aB2GP1) IgG, IgM, or IgA.
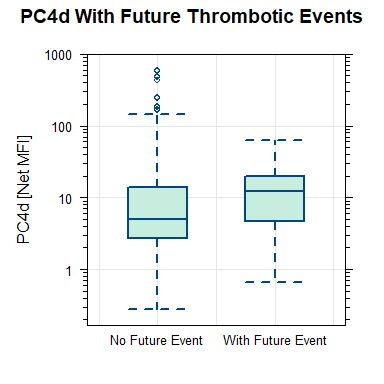 Figure 1. Box and whisker plot with PC4d levels (net MFI) in patients who experienced (right) or not (left) thrombotic events post-PC4d measurement.
Figure 1. Box and whisker plot with PC4d levels (net MFI) in patients who experienced (right) or not (left) thrombotic events post-PC4d measurement.
Disclosures: A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); J. Conklin, Exagen, Inc.; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; V. Kyttaris, Exagen, Corbus Pharmaceuticals, Aurinia Pharmaceuticals, Scipher; Y. Gartshteyn, None; W. Tang, None; A. Kammesheidt, Exagen Inc.; R. Alexander, Exagen Inc..
Background/Purpose: Platelet-bound complement activation products (PC4d) are associated with a history of thrombosis in systemic lupus erythematosus (SLE) (Gartshteyn al., 2021; Petri et al., 2017). The current study evaluated the role of PC4d in assessing the risk of future thrombosis.
Methods: PC4d (expressed at net mean fluorescence intensity [MFI]) was measured by flow cytometry from patients enrolled in the Lupus Cohorts at Columbia Univ (COL), Johns Hopkins Univ (JH) and Beth Israel Deaconess Medical Center (BI) between Apr-Sep 2017 (JH), Aug 2018-Jan 2020 (COL), and Oct 2018-Mar 2022 (BI). History of thrombotic vascular events was confirmed by medical record review. Data were analyzed by chi-square and logistic regression. Diagnostic odds ratio (DOR) was calculated. If a patient had 2 arterial or 2 venous events, only the closest in time to PC4d measurement was included in the analysis.
Results: A total of 419 SLE patients were enrolled. Main demographic and clinical characteristics are in Table 1. Seventy-four thrombotic events occurred in the 5 years pre- to 3 years post-PC4d measurement. Of these 74, 50% had PC4d≥10 MFI at enrollment while 72% of patients without thrombosis had PC4d< 10 (p< 0.001). 19 events occurred in 15 subjects in the 3 years after PC4d measurement: 8 cerebrovascular accidents, 3 deep vein thrombosis, 2 gastrointestinal infarctions, 2 myocardial infarctions, 1 pulmonary infarction, 1 arterial thrombosis and 2 venous thrombosis not specified. Median PC4d was higher in the 15 patients with than in the 404 patients without thrombosis (12.2 MFI, IQR: 4.7-19.6 vs. 4.9 MFI, IQR: 2.6-13.6, p=NS) (Figure 1). When the arterial events closest to PC4d measurements (8 of 14 total arterial events) were included, PC4d >13 MFI had sensitivity=62%, specificity=74%, DOR=4.8 (95%CI: 1.1, 20.3), p=0.034 in predicting future arterial thrombosis in patients with a previous arterial thrombosis. As expected, a previous arterial thrombosis was a strong predictor of a subsequent arterial event (p=0.0042). PC4d≥10 or ≥20 MFI did not reach statistical significance for future thrombosis neither in the entire population (p=0.055 and 0.51, respectively) nor in the subgroup younger than 65 years old (p = 0.99 for both). However, the negative predictive value of PC4d< 10 MFI was 98%, indicating that probability of not having a thrombosis within 3 years is 98% if PC4d< 10 MFI. Of the 285 patients with PC4d< 10 MFI, 105 (37%) were positive for at least 1 of 8 anti-phospholipid antibodies (aPL) (3 aCL, 3 anti-B2GP1, or 2 anti-PS/PT antibodies), and 22 (7.8%) were positive for 3 aPL, indicating that the probability of not having a thrombosis if PC4d< 10 MFI holds true for the population aPL positive and presumed at higher risk.
Conclusion: PC4d≥10 MFI is associated with thrombosis in SLE and predicts future arterial thrombosis (DOR=4.8), despite the small number of events post-PC4d. Interestingly, patients with PC4d < 10 MFI have a 98% probability of not experiencing a thrombotic event in the following 3 years. Taken together, these findings suggest that PC4d levels help evaluate the risk of thrombosis in SLE and can guide the decision to start low dose aspirin.
.gif) Table 1. Demographic characteristics of the patients in the 3 cohorts and overall.
Table 1. Demographic characteristics of the patients in the 3 cohorts and overall.Data is reported as number (n) and percentages (%). Anti-phospholipid (aPL) positivity was based on positivity for any of the isotypes: anti-phosphatidylserine/prothrombin complex (PS/PT) IgG or IgM, anti-cardiolipin (aCL) IgG, IgM, or IgA, and anti-beta2-glycoprotein1 (aB2GP1) IgG, IgM, or IgA.
 Figure 1. Box and whisker plot with PC4d levels (net MFI) in patients who experienced (right) or not (left) thrombotic events post-PC4d measurement.
Figure 1. Box and whisker plot with PC4d levels (net MFI) in patients who experienced (right) or not (left) thrombotic events post-PC4d measurement.Disclosures: A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); J. Conklin, Exagen, Inc.; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; V. Kyttaris, Exagen, Corbus Pharmaceuticals, Aurinia Pharmaceuticals, Scipher; Y. Gartshteyn, None; W. Tang, None; A. Kammesheidt, Exagen Inc.; R. Alexander, Exagen Inc..

