Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1166–1185) Systemic Sclerosis and Related Disorders – Basic Science Poster
1166: Scleroderma Fibroblasts Induce Alternative Macrophage Activation via an ERK1/2-dependent Mechanism
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AB
Andreea M. Bujor, MD
Boston University
Boston, MA, United States
Abstract Poster Presenter(s)
Taha Dinc1, Juan-Pablo Zertuche1, Fatima El Adili2, Maria Trojanowska3 and Andreea Bujor3, 1Boston University School of Medicine, Boston, MA, 2Boston University School of Medicine, Revere, MA, 3Boston University, Boston, MA
Background/Purpose: Activation of the innate immune system in systemic sclerosis (SSc), with an increased number of profibrotic/alternatively-activated macrophages in affected tissues, is an important aspect of disease pathogenesis. The exact mechanisms governing profibrotic macrophage activation and the potential crosstalk between resident fibroblasts and infiltrating macrophages have not been elucidated. MAPK/ERK1/2 signaling has been linked to inflammation and remodeling, but its role in alternative macrophage activation in SSc has not been explored.
Methods: Microarray analysis was performed using the Affymetrix GeneChip™human 2.0 ST gene array on macrophages stimulated with conditioned media from healthy control (HC) or SSc fibroblasts. Ingenuity Pathway Analysis (IPA) was carried out to see differentially regulated canonical pathways. THP1 cells were plated in direct co-culture with equal numbers of SSc or HC dermal fibroblasts. To inhibit signaling via ERK1/2, 5 µM of the SCH772984 specific ERK1/2 inhibitor was used. Activation of the ERK1/2 pathway was checked using pERk1/2 (Thr202/Tyr204, Cell Signaling) specific antibodies via Western blot. Quantitative real-time PCR was used to assess mRNA levels of profibrotic macrophage markers CD163 and CD204, with GAPDH as internal control.
Results: IPA analysis of human macrophages showed upregulated ERK/MAPK signaling pathway (p 0.0003). Western blot analyses of extracts from THP1 cocultured with SSc or HC fibroblasts showed higher levels of phosphorylated ERK1/2 in SSc vs HC fibroblast stimulated THP1 cells (Figure 1). Coculture of THP1 cells with fibroblasts induced increased expression of the alternatively-activated macrophage markers CD163 and CD204, with a more potent upregulation seen in response to SSc fibroblasts compared HC fibroblasts (9.5x vs 4.5x respectively compared to baseline, Figure 2). The effects on CD163 but not CD204 were abrogated by pretreatment with the ERK1/2 inhibitor.
Conclusion: In this study, we show that SSc fibroblasts induce increased CD163 expression in macrophages via an ERK1/2 dependent pathway, suggesting that crosstalk between fibroblasts and tissue resident macrophages may contribute to the alternative macrophage activation see in SSc.
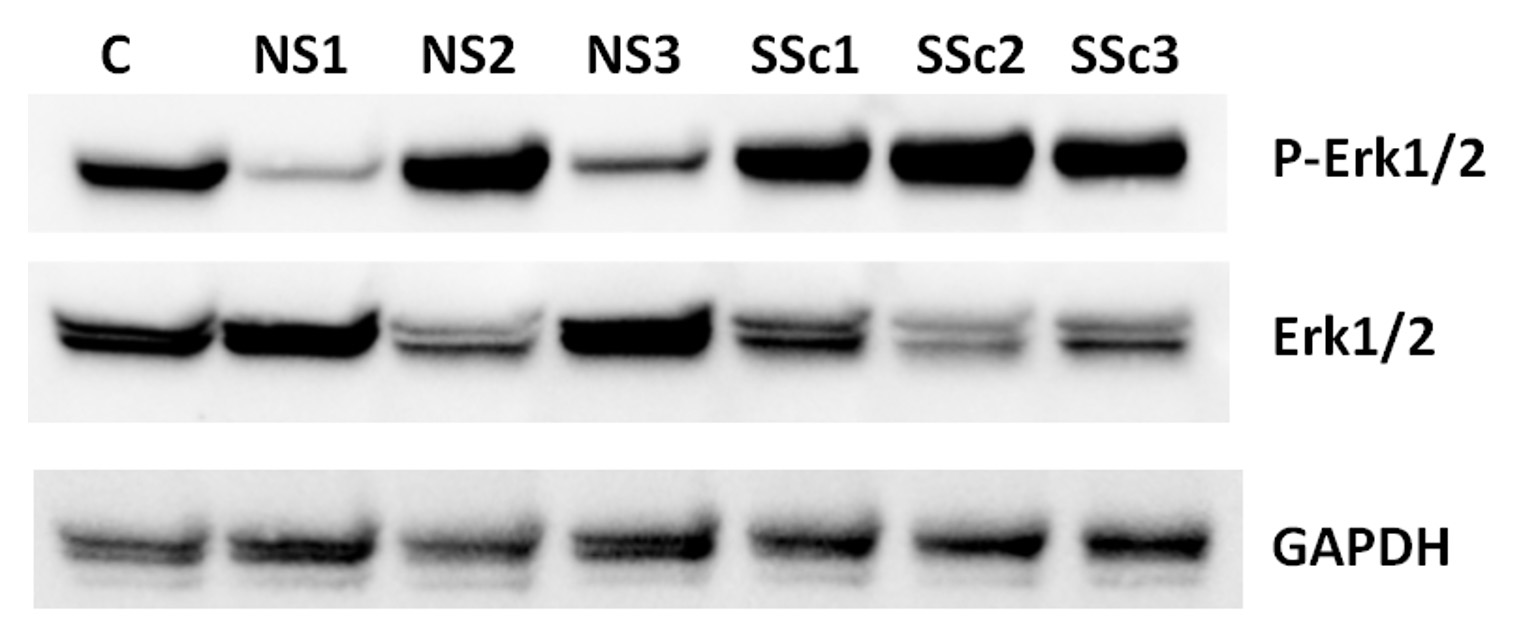 Figure 1. Western blot analysis of cell extracts from THP1 cells in coculture with NS (normal, healthy control) and SSc dermal fibroblasts showing increased phosphorylation of ERK1/2.
Figure 1. Western blot analysis of cell extracts from THP1 cells in coculture with NS (normal, healthy control) and SSc dermal fibroblasts showing increased phosphorylation of ERK1/2.
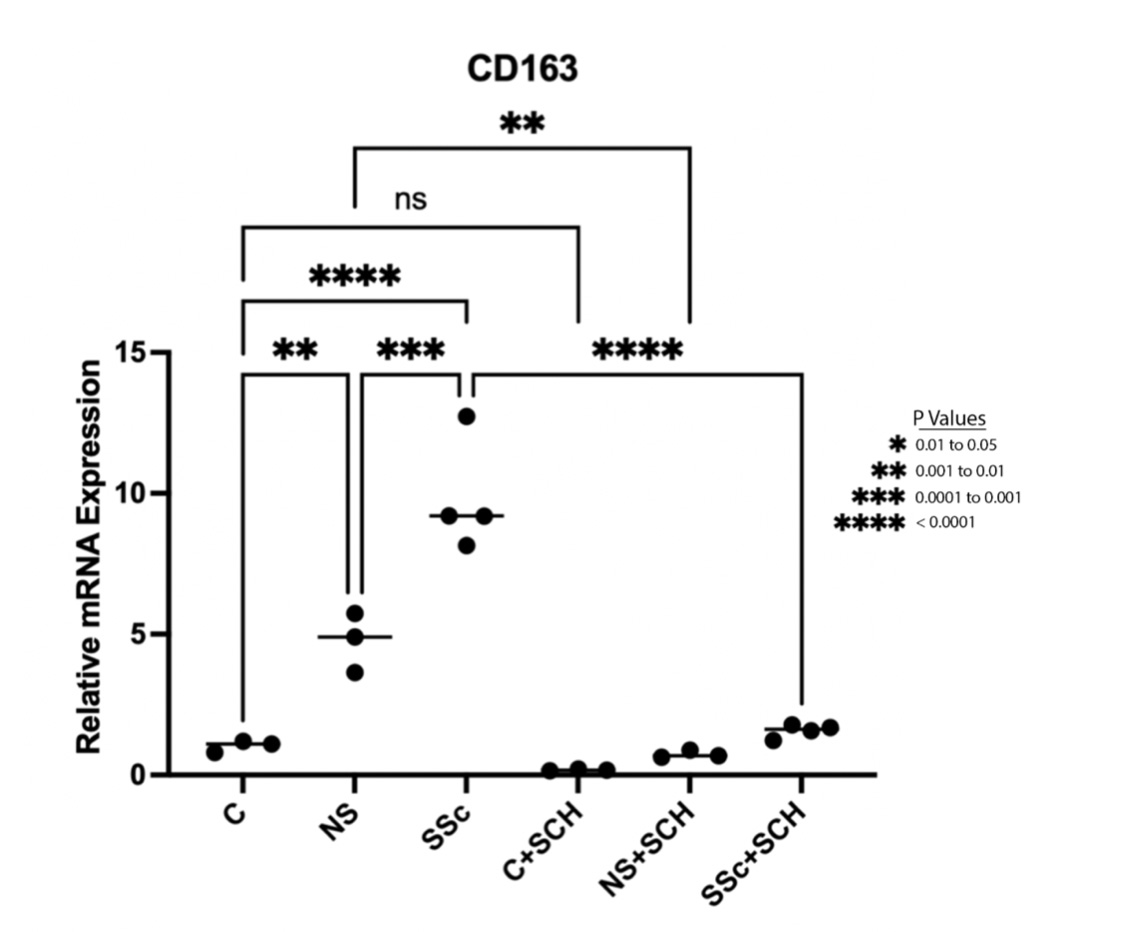 Figure 2. Increased CD163 mRNA levels in TJP1 cocultured with SSc and control fibroblasts. One way Anova (P < 0.0001), followed by Tukey’s multiple comparisons test was used for the analysis.
Figure 2. Increased CD163 mRNA levels in TJP1 cocultured with SSc and control fibroblasts. One way Anova (P < 0.0001), followed by Tukey’s multiple comparisons test was used for the analysis.
Disclosures: T. Dinc, None; J. Zertuche, None; F. El Adili, None; M. Trojanowska, None; A. Bujor, None.
Background/Purpose: Activation of the innate immune system in systemic sclerosis (SSc), with an increased number of profibrotic/alternatively-activated macrophages in affected tissues, is an important aspect of disease pathogenesis. The exact mechanisms governing profibrotic macrophage activation and the potential crosstalk between resident fibroblasts and infiltrating macrophages have not been elucidated. MAPK/ERK1/2 signaling has been linked to inflammation and remodeling, but its role in alternative macrophage activation in SSc has not been explored.
Methods: Microarray analysis was performed using the Affymetrix GeneChip™human 2.0 ST gene array on macrophages stimulated with conditioned media from healthy control (HC) or SSc fibroblasts. Ingenuity Pathway Analysis (IPA) was carried out to see differentially regulated canonical pathways. THP1 cells were plated in direct co-culture with equal numbers of SSc or HC dermal fibroblasts. To inhibit signaling via ERK1/2, 5 µM of the SCH772984 specific ERK1/2 inhibitor was used. Activation of the ERK1/2 pathway was checked using pERk1/2 (Thr202/Tyr204, Cell Signaling) specific antibodies via Western blot. Quantitative real-time PCR was used to assess mRNA levels of profibrotic macrophage markers CD163 and CD204, with GAPDH as internal control.
Results: IPA analysis of human macrophages showed upregulated ERK/MAPK signaling pathway (p 0.0003). Western blot analyses of extracts from THP1 cocultured with SSc or HC fibroblasts showed higher levels of phosphorylated ERK1/2 in SSc vs HC fibroblast stimulated THP1 cells (Figure 1). Coculture of THP1 cells with fibroblasts induced increased expression of the alternatively-activated macrophage markers CD163 and CD204, with a more potent upregulation seen in response to SSc fibroblasts compared HC fibroblasts (9.5x vs 4.5x respectively compared to baseline, Figure 2). The effects on CD163 but not CD204 were abrogated by pretreatment with the ERK1/2 inhibitor.
Conclusion: In this study, we show that SSc fibroblasts induce increased CD163 expression in macrophages via an ERK1/2 dependent pathway, suggesting that crosstalk between fibroblasts and tissue resident macrophages may contribute to the alternative macrophage activation see in SSc.
 Figure 1. Western blot analysis of cell extracts from THP1 cells in coculture with NS (normal, healthy control) and SSc dermal fibroblasts showing increased phosphorylation of ERK1/2.
Figure 1. Western blot analysis of cell extracts from THP1 cells in coculture with NS (normal, healthy control) and SSc dermal fibroblasts showing increased phosphorylation of ERK1/2. Figure 2. Increased CD163 mRNA levels in TJP1 cocultured with SSc and control fibroblasts. One way Anova (P < 0.0001), followed by Tukey’s multiple comparisons test was used for the analysis.
Figure 2. Increased CD163 mRNA levels in TJP1 cocultured with SSc and control fibroblasts. One way Anova (P < 0.0001), followed by Tukey’s multiple comparisons test was used for the analysis. Disclosures: T. Dinc, None; J. Zertuche, None; F. El Adili, None; M. Trojanowska, None; A. Bujor, None.

