Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0363: Centrally-Acting Angiotensin Converting Enzyme Inhibitor (cACEi) and Angiotensin Receptor Blocker (cARB) Use and Cognitive Dysfunction in Patients with Systemic Lupus
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- CD
Chrisanna Dobrowolski, MD, MS
Icahn School of Medicine at Mount Sinai
New York, NY, United States
Abstract Poster Presenter(s)
Chrisanna Dobrowolski1, Michelle Barraclough2, Jiandong Su3, Milica Tanic4, Kathleen Bingham5, Leslet Ruttan6, Dorcas Beaton7, Joan Wither8, Maria Carmela Tartaglia9, Mahta Kakvan3, Nicole Anderson3, Dennisse Bonilla3, Robin Green6 and Zahi Touma3, 1Icahn School of Medicine at Mount Sinai, New York, NY, 2Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, University of Toronto, Centre for Epidemiology Versus Arthritis, Division of Musculoskeletal and Dermatological Sciences, School of Biological Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Toronto, ON, Canada, 3Schroeder Arthritis Institute, Krembil Research Institute, University Health Network and University of Toronto, Toronto, ON, Canada, 4McMaster University, Toronto, ON, Canada, 5Centre for Mental Health, University Health Network; Department of Psychiatry, University of Toronto, Toronto, ON, Canada, 6University Health Network-Toronto Rehabilitation Institute, Toronto, ON, Canada, 7Institute for Work & Health, Toronto, ON, Canada, 8Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, University of Toronto, Toronto, ON, Canada, 9University of Toronto Krembil Neurosciences Centre, Toronto, ON, Canada
Background/Purpose: Cognitive dysfunction (CD) is detectable in 40% of patients with systemic lupus erythematosus (SLE). Despite this high prevalence, there are limited treatment options for this detrimental condition. Preliminary murine studies show potential for targeting microglial activation as a treatment of SLE-CD, which may be ameliorated with centrally-acting angiotensin converting enzyme inhibitor (cACEi) and angiotensin receptor blocker (cARB) use. The aim of this study is to determine if there is an association of cACEi/cARB use with SLE-CD in a 'real world', human SLE cohort.
Methods: The American College of Rheumatology (ACR) neuropsychological battery was administered to consecutive SLE patients at a single academic health center at 0, 6 and 12 months. Scores were compared to sex- and age-matched control subjects. Clinical and demographic data were gathered at each visit. The primary outcome was CD defined as dysfunction in ³2 cognitive domains. The primary predictor was total cumulative dose of cACEi/cARB in milligrams per kilogram (mg/kg), recorded as an equivalent Ramipril dose. Odds Ratio (OR) and 95% confidence interval (CI) of CD with respect to cACEi/cARB use was determined through generalized linear mixed modelling.
Results: 300 participants, meeting 2019 EULAR/ACR classification criteria for SLE, that represent 676 visits were included in the analysis. The majority of participants were female (89%), Caucasian (54%) and college or university educated (79%). Mean age at enrolment was 41±12 years, SLEDAI score (disease activity) 3.3±3.8 and SDI score (damage) (excluding CD) 1.0±1.4. Fifty-three (18%) participants were taking a cACEi or cARB, with a mean cumulative dose of 236mg/kg. One hundred sixteen participants (39%) met our criteria for CD. Caucasian ethnicity, current employment and higher cumulative azathioprine dose each had a protective effect with respect to CD; increasing Fatigue Severity Score was associated with higher odds of CD (Table 1). Use of cACEi/cARB and cACEi/cARB cumulative dose were both selected out of the multivariable model (Table 1). The null model (without any predictor) shows that 74% of the variability was explained by inter-patient differences in our study. Sensitivity analysis using propensity scores also failed to find a significant effect of cACEi/cARB on CD in SLE.
Conclusion: Our findings do not support the mechanistic literature, which suggests that cACEi and cARBs may be protective for CD associated with SLE. However, there are many limitations to our work. This study was designed retrospectively, based on data collected as part of a larger, longitudinal cohort study. As such, our sample size of those taking cACEi/cARBs was small and the cACEi/cARBs were mainly prescribed for hypertension. Hypertension has been linked to a risk of CD and so our study sample may have been biased. Despite these important limitations, this is the first study to examine the association between cACEi/cARB use and SLE-CD and is an important step to examining this potential therapeutic avenue for those with SLE-CD. Greater insight is anticipated from a randomized clinical trial which is currently underway.
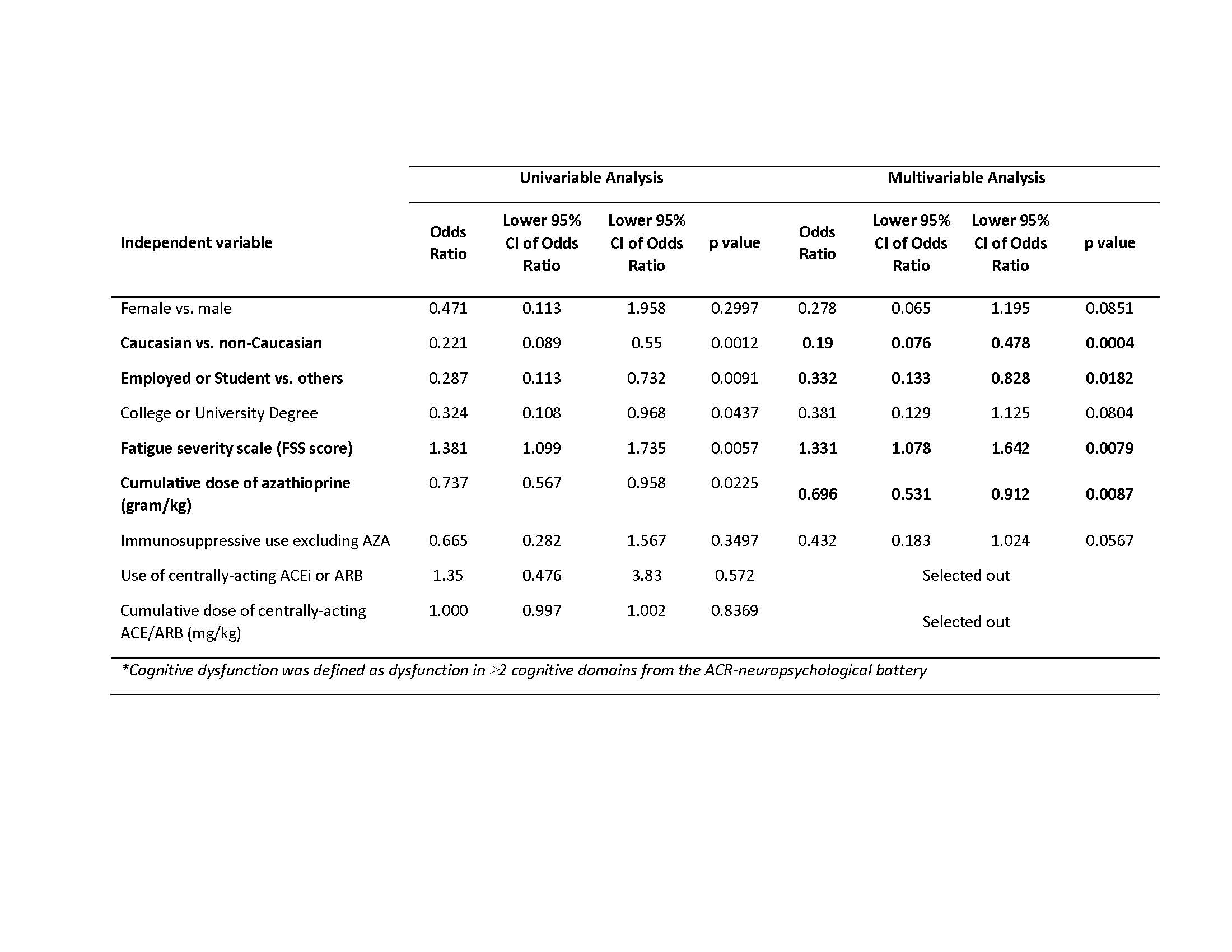 Table 1: Multivariable generalized linear mixed model: Cognitive dysfunction* versus select variables
Table 1: Multivariable generalized linear mixed model: Cognitive dysfunction* versus select variables
Disclosures: C. Dobrowolski, None; M. Barraclough, None; J. Su, None; M. Tanic, None; K. Bingham, None; L. Ruttan, None; D. Beaton, None; J. Wither, AstraZeneca, Pfizer; M. Tartaglia, None; M. Kakvan, None; N. Anderson, None; D. Bonilla, None; R. Green, None; Z. Touma, None.
Background/Purpose: Cognitive dysfunction (CD) is detectable in 40% of patients with systemic lupus erythematosus (SLE). Despite this high prevalence, there are limited treatment options for this detrimental condition. Preliminary murine studies show potential for targeting microglial activation as a treatment of SLE-CD, which may be ameliorated with centrally-acting angiotensin converting enzyme inhibitor (cACEi) and angiotensin receptor blocker (cARB) use. The aim of this study is to determine if there is an association of cACEi/cARB use with SLE-CD in a 'real world', human SLE cohort.
Methods: The American College of Rheumatology (ACR) neuropsychological battery was administered to consecutive SLE patients at a single academic health center at 0, 6 and 12 months. Scores were compared to sex- and age-matched control subjects. Clinical and demographic data were gathered at each visit. The primary outcome was CD defined as dysfunction in ³2 cognitive domains. The primary predictor was total cumulative dose of cACEi/cARB in milligrams per kilogram (mg/kg), recorded as an equivalent Ramipril dose. Odds Ratio (OR) and 95% confidence interval (CI) of CD with respect to cACEi/cARB use was determined through generalized linear mixed modelling.
Results: 300 participants, meeting 2019 EULAR/ACR classification criteria for SLE, that represent 676 visits were included in the analysis. The majority of participants were female (89%), Caucasian (54%) and college or university educated (79%). Mean age at enrolment was 41±12 years, SLEDAI score (disease activity) 3.3±3.8 and SDI score (damage) (excluding CD) 1.0±1.4. Fifty-three (18%) participants were taking a cACEi or cARB, with a mean cumulative dose of 236mg/kg. One hundred sixteen participants (39%) met our criteria for CD. Caucasian ethnicity, current employment and higher cumulative azathioprine dose each had a protective effect with respect to CD; increasing Fatigue Severity Score was associated with higher odds of CD (Table 1). Use of cACEi/cARB and cACEi/cARB cumulative dose were both selected out of the multivariable model (Table 1). The null model (without any predictor) shows that 74% of the variability was explained by inter-patient differences in our study. Sensitivity analysis using propensity scores also failed to find a significant effect of cACEi/cARB on CD in SLE.
Conclusion: Our findings do not support the mechanistic literature, which suggests that cACEi and cARBs may be protective for CD associated with SLE. However, there are many limitations to our work. This study was designed retrospectively, based on data collected as part of a larger, longitudinal cohort study. As such, our sample size of those taking cACEi/cARBs was small and the cACEi/cARBs were mainly prescribed for hypertension. Hypertension has been linked to a risk of CD and so our study sample may have been biased. Despite these important limitations, this is the first study to examine the association between cACEi/cARB use and SLE-CD and is an important step to examining this potential therapeutic avenue for those with SLE-CD. Greater insight is anticipated from a randomized clinical trial which is currently underway.
 Table 1: Multivariable generalized linear mixed model: Cognitive dysfunction* versus select variables
Table 1: Multivariable generalized linear mixed model: Cognitive dysfunction* versus select variablesDisclosures: C. Dobrowolski, None; M. Barraclough, None; J. Su, None; M. Tanic, None; K. Bingham, None; L. Ruttan, None; D. Beaton, None; J. Wither, AstraZeneca, Pfizer; M. Tartaglia, None; M. Kakvan, None; N. Anderson, None; D. Bonilla, None; R. Green, None; Z. Touma, None.

