Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0296: Characteristics and Treatment-selection in Patients with Rheumatoid Arthritis and with Inadequate Response to Janus Kinase Inhibitors
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- YM
Yusuke Miyazaki, MD, PhD
The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Japan
Kiakyusyu Fukuoka, Japan
Abstract Poster Presenter(s)
Yusuke Miyazaki1, Shingo Nakayamada2, Satoshi Kubo3, Koshiro Sonomoto2, Yoshino Inoue2, shunsuke fukuyo2, Kentaro Hanami2 and Yoshiya Tanaka4, 1The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Kiakyusyu Fukuoka, Japan, 2First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 3Department of Advanced Targeted Therapies, University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 4University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan
Background/Purpose: Janus kinase inhibitors (JAKinib) are effective in patients with rheumatoid arthritis (RA); however, some patients show an inadequate response to JAKinibs (JAKinib-IR). This study aimed to determine the clinical characteristics of JAKinib-IR patients with RA and identify suitable molecular-targeted drugs for such patients.
Methods: The subjects were 387 patients with RA and were recruited from the FIRST registry, a registry study of patients with RA receiving molecularly targeting therapies at multiple centers. The JAKinib (tofacitinib, 162 patients; baricitinib, 183; peficitinib, 8; upadacitinib, 30; and filgotinib, 4) were administered to the patients and the JAKinib-IR were defined by CDAI >10.0 at week52 despite treatments with JAKinibsm which were switched to a biologic DMARD or another JAK inhibitor. The efficacy and safety of switched molecular-targeted drugs were analyzed six months after switching treatment to identify suitable molecular-targeted drugs in JAKinib-IR patients.
Results: Overall, 70 (18.1%) patients were JAKinib-IR one year after the introduction of JAKinibs. The factors associated with JAKinibs-IR were identified only the numbers of previous biologics use (Odds ratio (OR) 1.26, 95% confidence interval (CI) 1.08-1.47, p=0.0041) by multivariable logistic regression analysis.Out of 70 JAKinib-IR patients who switched to another molecular-targeted drugs (TNFα inhibitor [TNFi], 16; non-TNFi, 26 (IL-6 receptor inhibitor, 19; abatacept, 7); JAKinib, 27), 12 (17.1%) patients achieved CDAI remission. There was no significant difference in the patient characteristics at the time of switching treatment among the three groups (group1: patients who switched to TNFi, group2: patients switched to non-TNFi, group3: patients switched to different JAKinib). The retention rates over 26 weeks did not differ among the three groups (group1, 75.0% (12/16); group2, 80.8% (21/26); group3, 85.7% (24/27), p=0.5766)There was no significant difference in the rate of adverse events leading to discontinuation of molecular target drugs among three groups. JAKinib-IR patients switching to TNFi did not exhibit a significant decrease in clinical disease activity index (CDAI) at 26 weeks. CDAI was improved at week 26 in JAKinib-IR patients switching to non-TNF inhibitor and a different JAKinib. The proportion of patients who achieved CDAI-remission was significantly higher in the group that switched to a different JAKinib (39.3%) than in the other drug groups (TNFi, 0.0%; non-TNFi, 3.9%; p< 0.001). The factors associated with CDAI-remission in JAKinib-IR patients were identified only switching to a different JAKinib (OR 27.57, 95%CI 3.08-246.6, p=0.003) by logistic regression analysis.
Conclusion: Seventy of 387 RA patients (18.1%) were JAKinib-IR in the FIRST registry. Only a number of prior bDMARDs at baseline affected JAKinib-IR by multi-variable analysis. Switch to another JAKinib was significantly more effective than switch to TNF-inhibitors in patients with the first JAKinib-IR.
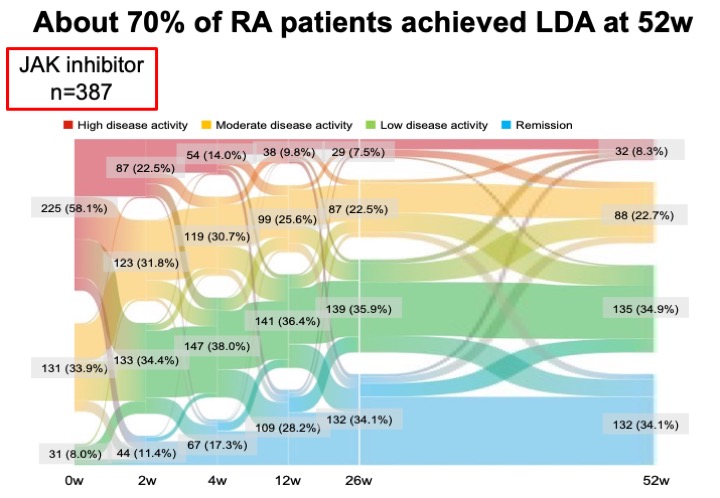 About 70% of RA patients achieved LDA at 52w .
About 70% of RA patients achieved LDA at 52w .
.jpeg) CDAI was improved at week 26 in JAKinib-IR patients switching to non-TNF inhibitor and a different JAK inhibitor.
CDAI was improved at week 26 in JAKinib-IR patients switching to non-TNF inhibitor and a different JAK inhibitor.
.jpeg) The proportion of patients who achieved remission was higher in the group that switched to a different JAKinibs
The proportion of patients who achieved remission was higher in the group that switched to a different JAKinibs
Disclosures: Y. Miyazaki, None; S. Nakayamada, None; S. Kubo, None; K. Sonomoto, None; Y. Inoue, None; s. fukuyo, None; K. Hanami, None; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly.
Background/Purpose: Janus kinase inhibitors (JAKinib) are effective in patients with rheumatoid arthritis (RA); however, some patients show an inadequate response to JAKinibs (JAKinib-IR). This study aimed to determine the clinical characteristics of JAKinib-IR patients with RA and identify suitable molecular-targeted drugs for such patients.
Methods: The subjects were 387 patients with RA and were recruited from the FIRST registry, a registry study of patients with RA receiving molecularly targeting therapies at multiple centers. The JAKinib (tofacitinib, 162 patients; baricitinib, 183; peficitinib, 8; upadacitinib, 30; and filgotinib, 4) were administered to the patients and the JAKinib-IR were defined by CDAI >10.0 at week52 despite treatments with JAKinibsm which were switched to a biologic DMARD or another JAK inhibitor. The efficacy and safety of switched molecular-targeted drugs were analyzed six months after switching treatment to identify suitable molecular-targeted drugs in JAKinib-IR patients.
Results: Overall, 70 (18.1%) patients were JAKinib-IR one year after the introduction of JAKinibs. The factors associated with JAKinibs-IR were identified only the numbers of previous biologics use (Odds ratio (OR) 1.26, 95% confidence interval (CI) 1.08-1.47, p=0.0041) by multivariable logistic regression analysis.Out of 70 JAKinib-IR patients who switched to another molecular-targeted drugs (TNFα inhibitor [TNFi], 16; non-TNFi, 26 (IL-6 receptor inhibitor, 19; abatacept, 7); JAKinib, 27), 12 (17.1%) patients achieved CDAI remission. There was no significant difference in the patient characteristics at the time of switching treatment among the three groups (group1: patients who switched to TNFi, group2: patients switched to non-TNFi, group3: patients switched to different JAKinib). The retention rates over 26 weeks did not differ among the three groups (group1, 75.0% (12/16); group2, 80.8% (21/26); group3, 85.7% (24/27), p=0.5766)There was no significant difference in the rate of adverse events leading to discontinuation of molecular target drugs among three groups. JAKinib-IR patients switching to TNFi did not exhibit a significant decrease in clinical disease activity index (CDAI) at 26 weeks. CDAI was improved at week 26 in JAKinib-IR patients switching to non-TNF inhibitor and a different JAKinib. The proportion of patients who achieved CDAI-remission was significantly higher in the group that switched to a different JAKinib (39.3%) than in the other drug groups (TNFi, 0.0%; non-TNFi, 3.9%; p< 0.001). The factors associated with CDAI-remission in JAKinib-IR patients were identified only switching to a different JAKinib (OR 27.57, 95%CI 3.08-246.6, p=0.003) by logistic regression analysis.
Conclusion: Seventy of 387 RA patients (18.1%) were JAKinib-IR in the FIRST registry. Only a number of prior bDMARDs at baseline affected JAKinib-IR by multi-variable analysis. Switch to another JAKinib was significantly more effective than switch to TNF-inhibitors in patients with the first JAKinib-IR.
 About 70% of RA patients achieved LDA at 52w .
About 70% of RA patients achieved LDA at 52w ..jpeg) CDAI was improved at week 26 in JAKinib-IR patients switching to non-TNF inhibitor and a different JAK inhibitor.
CDAI was improved at week 26 in JAKinib-IR patients switching to non-TNF inhibitor and a different JAK inhibitor..jpeg) The proportion of patients who achieved remission was higher in the group that switched to a different JAKinibs
The proportion of patients who achieved remission was higher in the group that switched to a different JAKinibsDisclosures: Y. Miyazaki, None; S. Nakayamada, None; S. Kubo, None; K. Sonomoto, None; Y. Inoue, None; s. fukuyo, None; K. Hanami, None; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly.

