Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1332–1359) Patient Outcomes, Preferences, and Attitudes Poster II
1357: The ANCA-Associated Vasculitis Patient-Reported Outcome (AAV-PRO) Captures Depression and Health-Related Quality of Life More Than Disease Activity and Damage
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AM
Annika Maunz, -None-
Eberhard Karls University of Tübingen
Tübingen, Germany
Abstract Poster Presenter(s)
Annika Maunz1, Joerg Henes2, Joanna Robson3, Johann Jacoby4, Bernhard Hellmich5 and Christian Löffler6, 1Medius Kliniken - Teaching Hospital University of Tübingen, Kirchheim-Teck, Tübingen, Germany, 2Center for Interdisciplinary Clinical Immunology, Rheumatology and Auto-inflammatory Diseases (INDIRA), University Hospital Tübingen, Tübingen, Germany, 3UWE Bristol, Bristol, United Kingdom, 4University Hospital Tübingen, Institute for Clinical Epidemiology and Applied Biometry, Tübingen, Germany, 5Klinik für Innere Medizin, Rheumatologie & Immunologie, Medius Kliniken, Universität Tübingen, Plochingen, Germany, 6Medius Kliniken - Teaching Hospital University of Tübingen,, Kirchheim Teck, Germany
Background/Purpose: The ANCA-associated vasculitis patient-reported outcome (AAV-PRO) is a new disease-specific PRO developed to capture the impact of having AAV and dealing with its treatment (Robson et al., 2018). It therefore may be more specific to health-related quality of life (HRQoL) in this disease than generic measures. As a profile measure the AAV-PRO contains six different domains: Organ-specific symptoms (OSS), systemic symptoms severity (SSS), treatment side effects (TSE), social and emotional impact (SEI), concerns about the future (CAF), and physical function (PF). The aim of this study was to explore the association of disease activity, damage, depression, HRQoL, treatment, relapses, and organ manifestations with the patient's AAV-PRO domain scores.
Methods: AAV-PRO, Beck's depression inventory (BDI), Short Form 36 (SF36), Birmingham Vasculitis Activity Score (BVAS) and Vasculitis Damage Index (VDI) were completed at baseline (t1) and after 3-6 months (t2). In addition, patient data such as age, diagnosis, therapies, relapses, and organ manifestations were recorded. Data were analyzed by t-tests and correlation-based regression analyses.
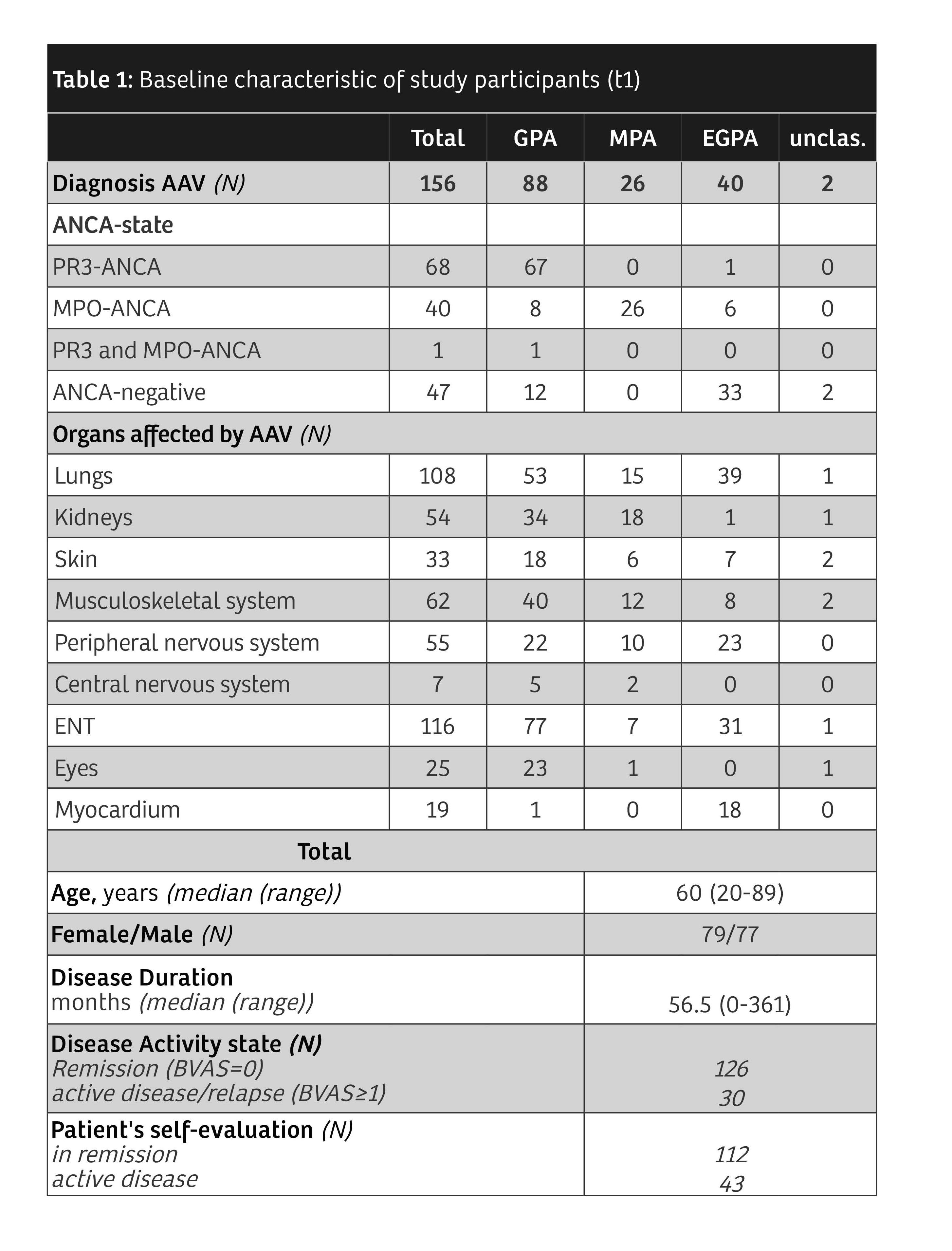
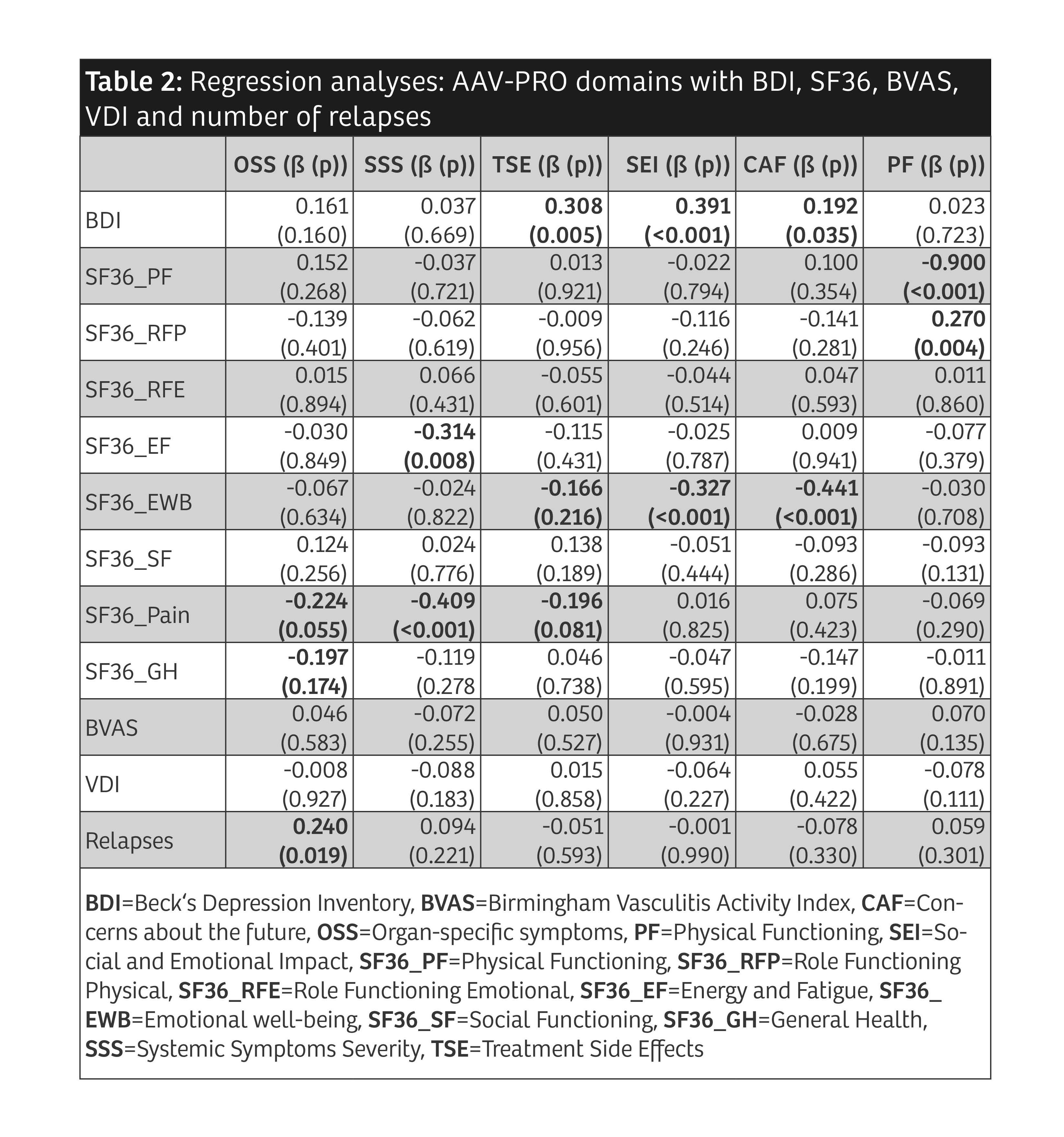
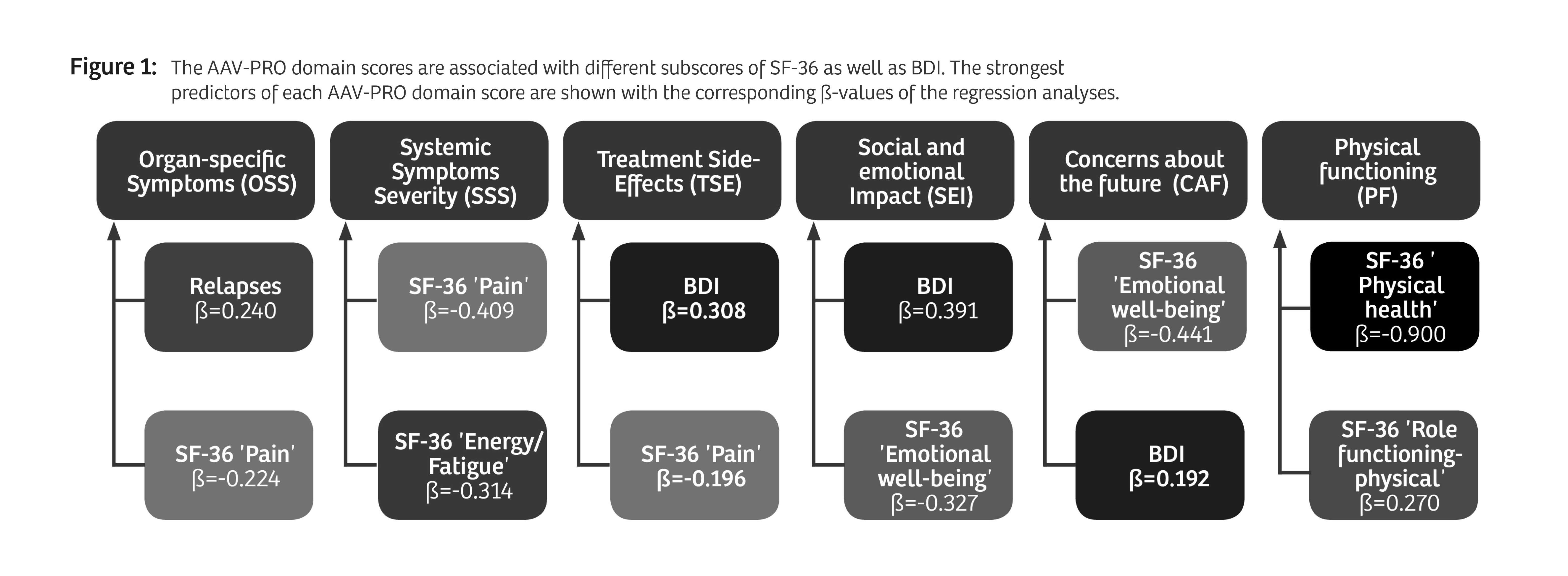
Results: 156 patients with AAV participated (table 1). Median AAV-PRO domain scores were higher in patients reporting "active disease" compared to patients reporting "in remission" (p< 0.001). When the patient cohort was divided based on objective disease activity (BVAS ≥ 1) the results of mean differences were heterogenous. The strongest predictors found in the regression analyses for each AAV-PRO domain are depicted in Figure 1. Detailed results are shown in Table 2. OSS was mainly predicted by SF36 'pain' and the number of relapses. For SSS, it was SF36 'pain' and 'energy/fatigue'. The strongest predictor of TSE was the BDI. SEI and CAF were chiefly predicted by the BDI and SF36 'emotional well-being'. Overall, the strongest association was seen between PF and SF36 'physical functioning'. In regression analyses predicting the BDI by the AAV-PRO domains, SEI was found to have high predictive power for the BDI. Substantial relationships were also found when regressing the SF36 subdomains on the AAV-PRO domains. There were no significant relationships between the AAV-PRO domains and BVAS, VDI, therapies or organ manifestations. This pattern of results held both at t1 and t2.
Conclusion: We confirm the previously reported strong association of AAV-PRO domain scoring to the patient's self-evaluation in a German AAV cohort. Our data show convergent validity of all AAV-PRO subdomains with the established questionnaires BDI and SF-36. However, none of the AAV-PRO domains are markedly related to objective parameters of disease such as BVAS and VDI. Thus, we regard the AAV-PRO as a valuable addition that might complement traditional endpoints like activity and damage in future clinical trials. The replicability of the current results should be assessed in the future, possibly with patients with more active vasculitis and with a larger sample that provides more power.
Disclosures: A. Maunz, None; J. Henes, Boehringer Ingelheim; J. Robson, None; J. Jacoby, None; B. Hellmich, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, GlaxoSmithKline (GSK), InflaRx, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, Vifor; C. Löffler, None.
Background/Purpose: The ANCA-associated vasculitis patient-reported outcome (AAV-PRO) is a new disease-specific PRO developed to capture the impact of having AAV and dealing with its treatment (Robson et al., 2018). It therefore may be more specific to health-related quality of life (HRQoL) in this disease than generic measures. As a profile measure the AAV-PRO contains six different domains: Organ-specific symptoms (OSS), systemic symptoms severity (SSS), treatment side effects (TSE), social and emotional impact (SEI), concerns about the future (CAF), and physical function (PF). The aim of this study was to explore the association of disease activity, damage, depression, HRQoL, treatment, relapses, and organ manifestations with the patient's AAV-PRO domain scores.
Methods: AAV-PRO, Beck's depression inventory (BDI), Short Form 36 (SF36), Birmingham Vasculitis Activity Score (BVAS) and Vasculitis Damage Index (VDI) were completed at baseline (t1) and after 3-6 months (t2). In addition, patient data such as age, diagnosis, therapies, relapses, and organ manifestations were recorded. Data were analyzed by t-tests and correlation-based regression analyses.
Results: 156 patients with AAV participated (table 1). Median AAV-PRO domain scores were higher in patients reporting "active disease" compared to patients reporting "in remission" (p< 0.001). When the patient cohort was divided based on objective disease activity (BVAS ≥ 1) the results of mean differences were heterogenous. The strongest predictors found in the regression analyses for each AAV-PRO domain are depicted in Figure 1. Detailed results are shown in Table 2. OSS was mainly predicted by SF36 'pain' and the number of relapses. For SSS, it was SF36 'pain' and 'energy/fatigue'. The strongest predictor of TSE was the BDI. SEI and CAF were chiefly predicted by the BDI and SF36 'emotional well-being'. Overall, the strongest association was seen between PF and SF36 'physical functioning'. In regression analyses predicting the BDI by the AAV-PRO domains, SEI was found to have high predictive power for the BDI. Substantial relationships were also found when regressing the SF36 subdomains on the AAV-PRO domains. There were no significant relationships between the AAV-PRO domains and BVAS, VDI, therapies or organ manifestations. This pattern of results held both at t1 and t2.
Conclusion: We confirm the previously reported strong association of AAV-PRO domain scoring to the patient's self-evaluation in a German AAV cohort. Our data show convergent validity of all AAV-PRO subdomains with the established questionnaires BDI and SF-36. However, none of the AAV-PRO domains are markedly related to objective parameters of disease such as BVAS and VDI. Thus, we regard the AAV-PRO as a valuable addition that might complement traditional endpoints like activity and damage in future clinical trials. The replicability of the current results should be assessed in the future, possibly with patients with more active vasculitis and with a larger sample that provides more power.



Disclosures: A. Maunz, None; J. Henes, Boehringer Ingelheim; J. Robson, None; J. Jacoby, None; B. Hellmich, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, GlaxoSmithKline (GSK), InflaRx, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, Vifor; C. Löffler, None.

