Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1387–1416) RA – Diagnosis, Manifestations, and Outcomes Poster III
1388: Updating and Validating the Rheumatic Disease Comorbidity Index to Incorporate ICD-10 Diagnostic Codes
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AD
Anthony Dolomisiewicz, MD
University of Nebraska Medical Center
Omaha, NE, United States
Abstract Poster Presenter(s)
Anthony Dolomisiewicz1, Hanifah Ali2, Punyasha Roul3, Yangyuna Yang1, Grant Cannon4, Joshua Baker5, Ted Mikuls6, Kaleb Michaud1 and Bryant England1, 1University of Nebraska Medical Center, Omaha, NE, 2UNMC, Omaha, 3UNMC, Omaha, NE, 4Salt Lake City VA, Salt Lake city, 5University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, 6Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: The Rheumatic Disease Comorbidity Index (RDCI) was designed to quantify comorbidity burden for risk stratification of health outcomes in patients with rheumatic disease. Developed from self-reported conditions and previously validated using ICD-9 codes, the RDCI has yet to be adapted to the ICD-10 coding system despite regular use for outcomes research in rheumatology. We aimed to develop, and validate the performance of, an ICD-10 code list for the RDCI.
Methods: We defined ICD-9 and ICD-10 era cohorts spanning the transition from ICD-9 to ICD-10 within the Veterans Affairs Rheumatoid Arthritis Registry, a multicenter prospective cohort of U.S. Veterans with RA. Comorbidities were collected from linked, national VA administrative data over a two-year period in both cohorts using existing ICD-9 codes and a new ICD-10 code list generated from diagnosis crosswalks and clinical expertise. Over a subsequent two-year follow-up period, MDHAQ scores were obtained from the registry and mortality was ascertained from linked VA death records. ICD-9 and ICD-10 derived comorbidity frequencies and RDCI scores were compared using Cohen's Kappa and Intraclass Correlation Coefficients (ICC). The ability of the RDCI to improve the prediction of functional status and mortality was assessed using multivariable regression models and goodness of fit statistics (AIC, QICu).
Results: The ICD-9 (n=1,082) and ICD-10 (n=1,445) cohorts were predominantly male (89%, 87%), Caucasian (77%, 74%), and middle to older-aged (mean 67 years, 68 years). Mean (SD) RDCI scores were 2.95 (1.73) for ICD-9 and 2.93 (1.75) for ICD-10 cohorts (Table 1). Among individuals observed during both ICD-9 and ICD-10 eras (n=862), RDCI scores had moderate agreement (ICC 0.71 [0.68-0.74]). Prevalence of comorbidities were similar between coding systems with absolute differences less than 4% (range: 0.3 to 3.9%). Myocardial infarction, hypertension, diabetes mellitus, depression, stroke, other cardiovascular, lung disease, and cancer had moderate agreement or higher (range κ: 0.47 to 0.84), while fracture and ulcer/stomach problem had slight and fair agreement (κ=0.13; κ=0.27) (Table 1). Higher RDCI scores were associated with a greater risk of death, and model performance improved with the addition of the RDCI score in both ICD-9 and ICD-10 cohorts (Table 2). The reduction in AIC (better model performance) was greater in the ICD-10 cohort than ICD-9 (fully adjusted models -23.02 vs. -1.11). Higher RDCI scores were also associated with worse functional status (Table 3). A greater reduction in QICu (better model performance) was seen in the ICD-10 than the ICD-9 cohort with the addition of the RDCI scores to the models predicting MDHAQ (fully adjusted models -60.79 vs. -21.09).
Conclusion: The newly proposed ICD-10 codes for the RDCI generated comparable RDCI scores and chronic disease prevalence estimates to those derived from previously validated ICD-9 codes. RDCI scores calculated using ICD-10 codes are highly predictive of functional status and mortality, comparing favorably to scores based on ICD-9 codes. The proposed ICD-10 codes for the RDCI can be used in outcomes research spanning the ICD-10 era.
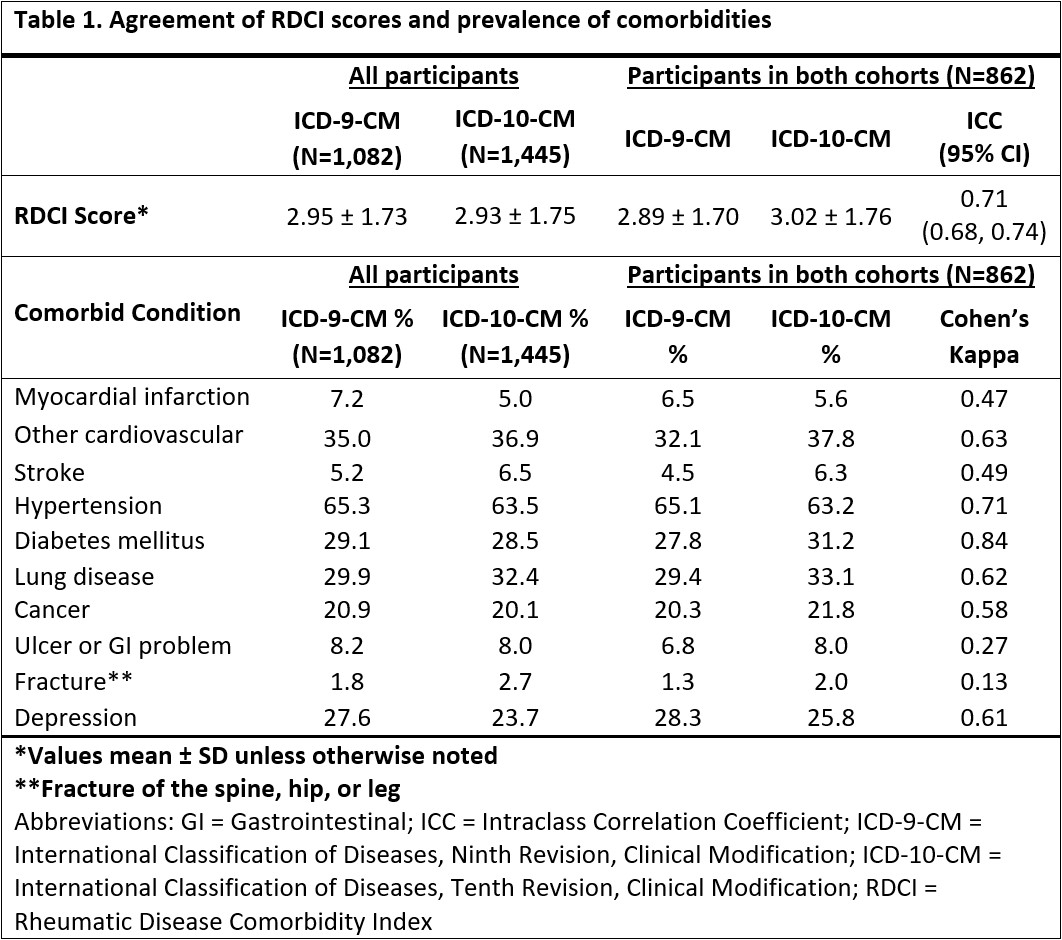 Table 1. Agreement of RDCI scores and prevalence of comorbidities
Table 1. Agreement of RDCI scores and prevalence of comorbidities
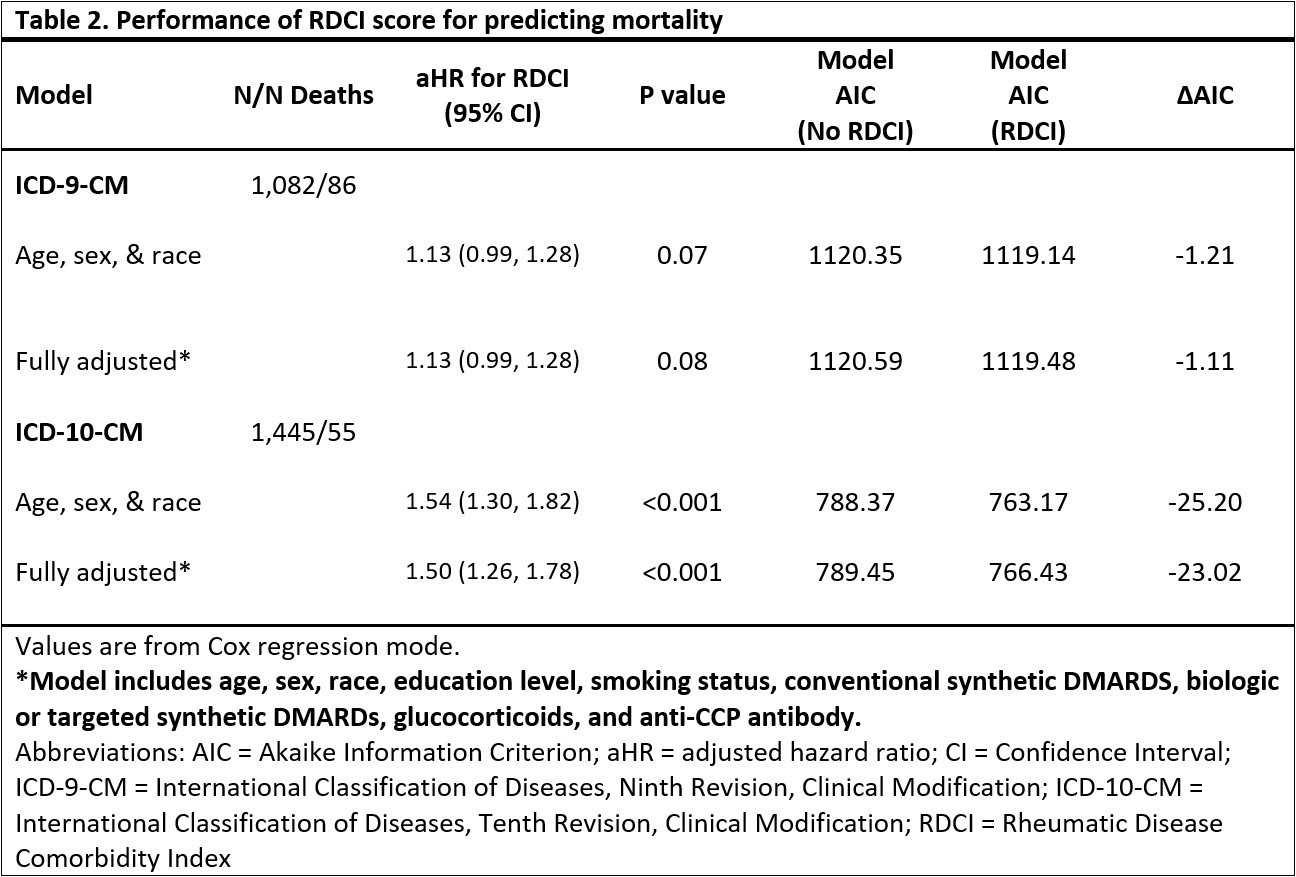 Table 2. Performance of RDCI score for predicting mortality
Table 2. Performance of RDCI score for predicting mortality
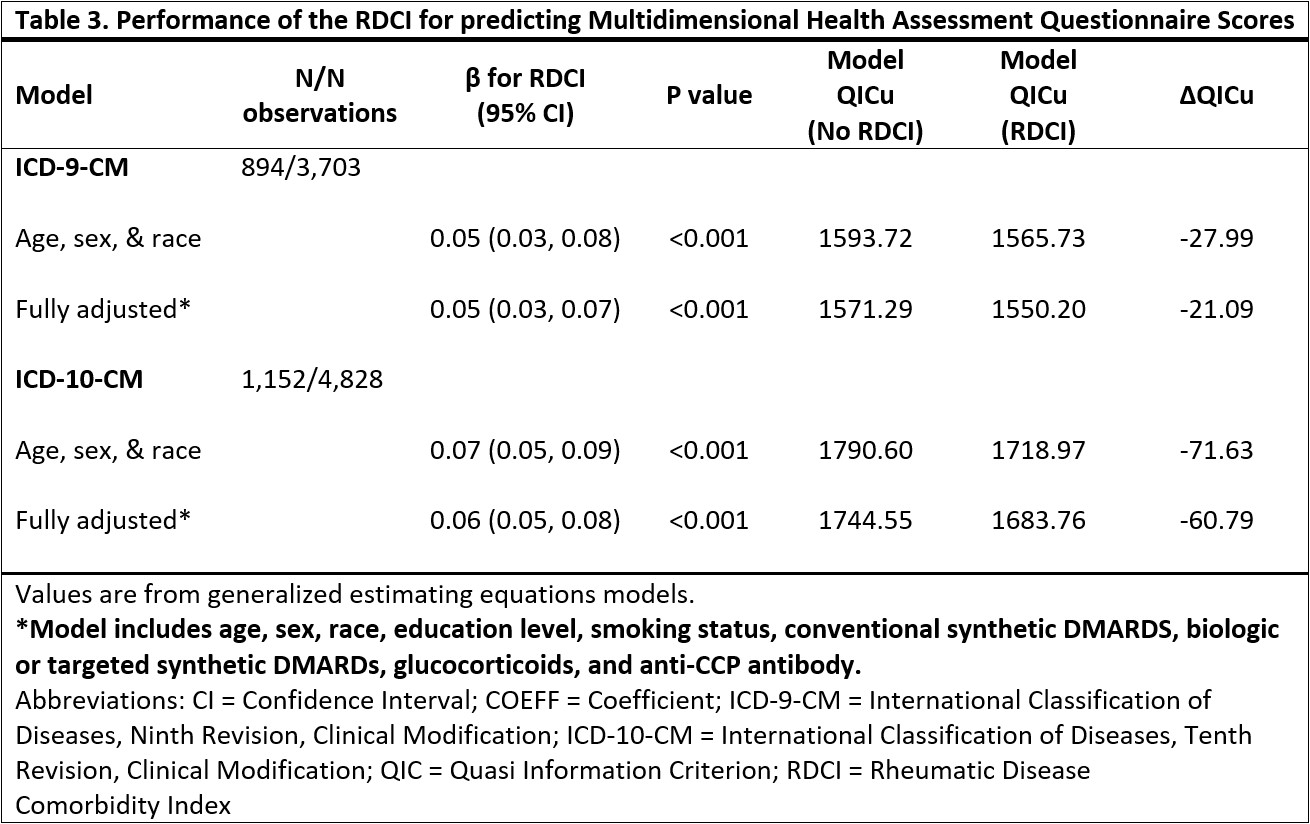 Table 3. Performance of the RDCI for predicting Multidimensional Health Assessment Questionnaire Scores
Table 3. Performance of the RDCI for predicting Multidimensional Health Assessment Questionnaire Scores
Disclosures: A. Dolomisiewicz, None; H. Ali, None; P. Roul, None; Y. Yang, None; G. Cannon, None; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; K. Michaud, None; B. England, Boehringer-Ingelheim.
Background/Purpose: The Rheumatic Disease Comorbidity Index (RDCI) was designed to quantify comorbidity burden for risk stratification of health outcomes in patients with rheumatic disease. Developed from self-reported conditions and previously validated using ICD-9 codes, the RDCI has yet to be adapted to the ICD-10 coding system despite regular use for outcomes research in rheumatology. We aimed to develop, and validate the performance of, an ICD-10 code list for the RDCI.
Methods: We defined ICD-9 and ICD-10 era cohorts spanning the transition from ICD-9 to ICD-10 within the Veterans Affairs Rheumatoid Arthritis Registry, a multicenter prospective cohort of U.S. Veterans with RA. Comorbidities were collected from linked, national VA administrative data over a two-year period in both cohorts using existing ICD-9 codes and a new ICD-10 code list generated from diagnosis crosswalks and clinical expertise. Over a subsequent two-year follow-up period, MDHAQ scores were obtained from the registry and mortality was ascertained from linked VA death records. ICD-9 and ICD-10 derived comorbidity frequencies and RDCI scores were compared using Cohen's Kappa and Intraclass Correlation Coefficients (ICC). The ability of the RDCI to improve the prediction of functional status and mortality was assessed using multivariable regression models and goodness of fit statistics (AIC, QICu).
Results: The ICD-9 (n=1,082) and ICD-10 (n=1,445) cohorts were predominantly male (89%, 87%), Caucasian (77%, 74%), and middle to older-aged (mean 67 years, 68 years). Mean (SD) RDCI scores were 2.95 (1.73) for ICD-9 and 2.93 (1.75) for ICD-10 cohorts (Table 1). Among individuals observed during both ICD-9 and ICD-10 eras (n=862), RDCI scores had moderate agreement (ICC 0.71 [0.68-0.74]). Prevalence of comorbidities were similar between coding systems with absolute differences less than 4% (range: 0.3 to 3.9%). Myocardial infarction, hypertension, diabetes mellitus, depression, stroke, other cardiovascular, lung disease, and cancer had moderate agreement or higher (range κ: 0.47 to 0.84), while fracture and ulcer/stomach problem had slight and fair agreement (κ=0.13; κ=0.27) (Table 1). Higher RDCI scores were associated with a greater risk of death, and model performance improved with the addition of the RDCI score in both ICD-9 and ICD-10 cohorts (Table 2). The reduction in AIC (better model performance) was greater in the ICD-10 cohort than ICD-9 (fully adjusted models -23.02 vs. -1.11). Higher RDCI scores were also associated with worse functional status (Table 3). A greater reduction in QICu (better model performance) was seen in the ICD-10 than the ICD-9 cohort with the addition of the RDCI scores to the models predicting MDHAQ (fully adjusted models -60.79 vs. -21.09).
Conclusion: The newly proposed ICD-10 codes for the RDCI generated comparable RDCI scores and chronic disease prevalence estimates to those derived from previously validated ICD-9 codes. RDCI scores calculated using ICD-10 codes are highly predictive of functional status and mortality, comparing favorably to scores based on ICD-9 codes. The proposed ICD-10 codes for the RDCI can be used in outcomes research spanning the ICD-10 era.
 Table 1. Agreement of RDCI scores and prevalence of comorbidities
Table 1. Agreement of RDCI scores and prevalence of comorbidities Table 2. Performance of RDCI score for predicting mortality
Table 2. Performance of RDCI score for predicting mortality Table 3. Performance of the RDCI for predicting Multidimensional Health Assessment Questionnaire Scores
Table 3. Performance of the RDCI for predicting Multidimensional Health Assessment Questionnaire ScoresDisclosures: A. Dolomisiewicz, None; H. Ali, None; P. Roul, None; Y. Yang, None; G. Cannon, None; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; K. Michaud, None; B. England, Boehringer-Ingelheim.

