Back
Poster Session C
Systemic lupus erythematosus (SLE)
Session: (1440–1485) SLE – Diagnosis, Manifestations, and Outcomes Poster II: Manifestations
1478: Urine ALCAM Is a Strong Predictor of Lupus Nephritis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- DC
Dalena Chu, MSc
Equillium, Inc.
La Jolla, CA, United States
Abstract Poster Presenter(s)
Dalena Chu1, Noa Schwartz2, Jeanette Ampudia1, Joel Guthridge3, Judith James3, Jill Buyon4, Stephen Connelly1, Maple Fung1, Cherie Ng1, AMP SLE/RA consortium5, Michelle Petri6, Chandra Mohan7 and Chaim Putterman8, 1Equillium, Inc., La Jolla, CA, 2Albert Einstein College of Medicine/Montefiore Medical Center, New York, NY, 3Oklahoma Medical Research Foundation, Oklahoma City, OK, 4NYU Grossman School of Medicine, New York, NY, 5Accelerating Medicines Partnership, Los Angeles, CA, 6Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 7University of Houston, Houston, TX, 8Albert Einstein College of Medicine, Bronx, NY
Background/Purpose: T cells play a critical role in the pathogenicity of SLE and lupus nephritis (LN). Hence, identifying T cell co-stimulatory pathways and mediators that correlate with disease activity may significantly aid in the development of biomarkers applicable for clinical use, and help identify novel therapeutic targets. We found that expression of CD6 (on T cells) and ALCAM (on antigen presenting cells and on tubular cells) is upregulated in the kidneys in LN mouse models and that blockade of the pathway using an anti-CD6 monoclonal antibody significantly reduced the severity of kidney involvement, implicating an important role for the CD6-ALCAM pathway in the development of LN. Furthermore, we found that soluble urine ALCAM is significantly elevated in patients with LN. Here, we expand upon the previous analyses by evaluating urine ALCAM as a predictor of nephritis progression and/or resolution.
Methods: Patient urine and serum samples were acquired through the Accelerating Medicines Partnership (AMP), a public-private partnership to accelerate discovery and development of therapeutics for autoimmune diseases. Samples were obtained from patients with biopsy proven LN and living kidney donor controls and longitudinal sampling (0, 3, 6, and 12 months) was available for 143 LN patients. Soluble levels of ALCAM were quantitated by ELISA, CD6 levels by a proprietary ECL assay, and urine creatinine from the same urine sample was measured by an improved Jaffe method. Urine ALCAM or CD6 levels were then normalized to urine creatinine levels on a pg (ALCAM or CD6) per mg creatinine basis. Responder status (complete response (CR), partial response (PR), no response (NR)) was as determined by AMP. In a separate analysis, responder and non-responder status was determined by change in UPCR where responders showed a decrease in UPCR of greater than 50% from the selected starting point.
Results: Cross-sectional analysis at baseline/week 0 showed that urine ALCAM significantly correlated with renal-SLEDAI (r(250) = 0.2460, p< 0.0001), the Physician Global Assessment (PGA) score (r(198) = 0.3325, p< 0.0001), and negatively with serum C3 (r(255) = -0.2170, p< 0.001) and C4 (r(253) = -0.1368, p< 0.05) (Figure 1). A receiver operating characteristic (ROC) curve was used to assess the sensitivity and specificity of urine ALCAM and CD6 as predictors of LN compared to healthy controls. Urine ALCAM is a strong predictor of active LN with an area under the curve (AUC) of 0.9781, compared to urine CD6 with an AUC of 0.7168. To evaluate longitudinal changes in urine ALCAM or CD6 and their associations with response status, the change between week 0 and week 26 was determined and related back to response status. Partial and complete responders experienced a significant reduction in urine ALCAM compared to non-responders, irrespective of the type of treatment, while urine CD6 did not distinguish between these groups (Figure 2).
Conclusion: Urinary ALCAM is a biomarker of disease severity in LN that could be indicative of a patient's response to treatment. Analyses are in progress to evaluate ALCAM in combination with other measures of disease severity to predict longer term disease outcomes.
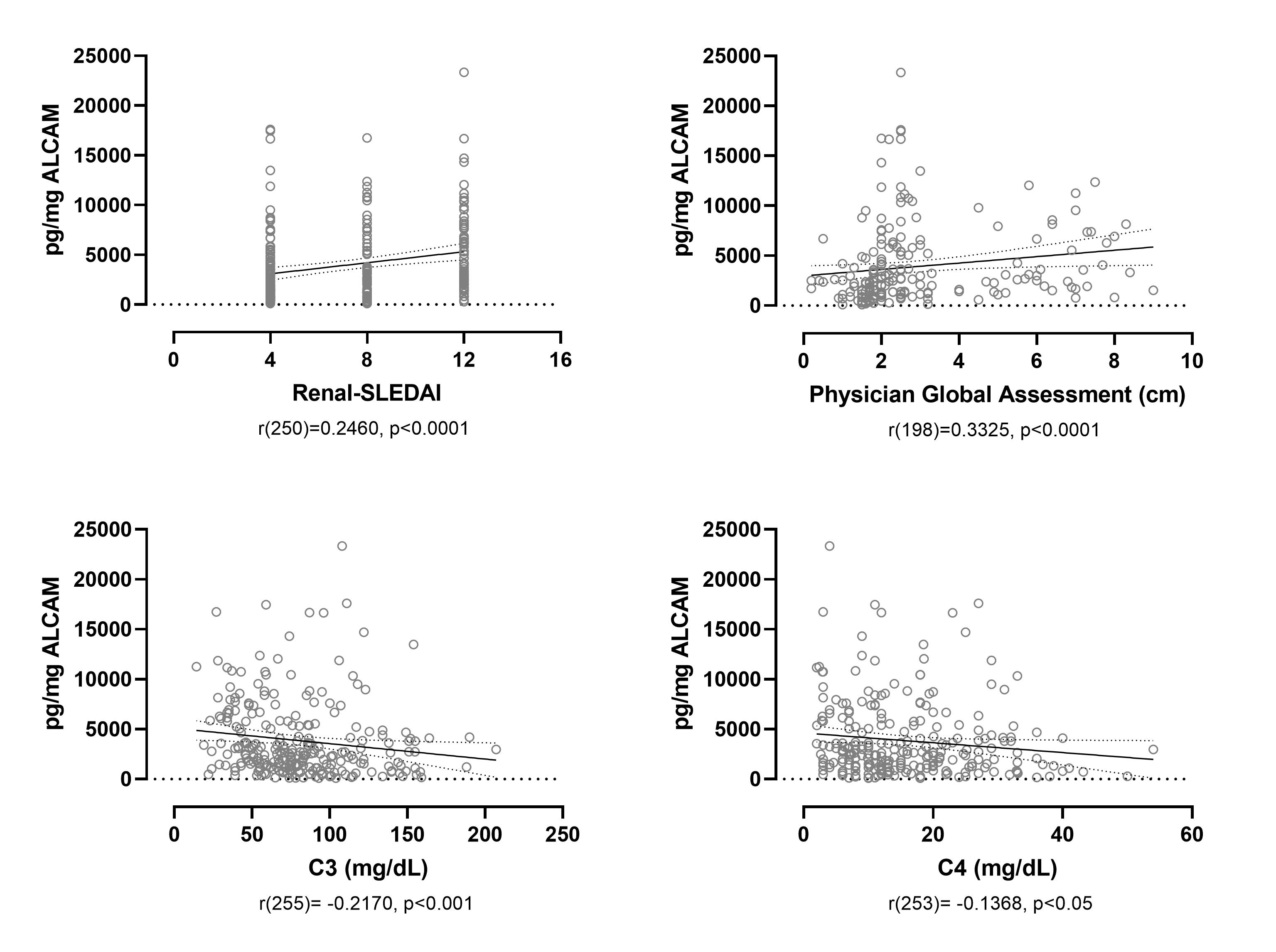 Urine ALCAM significantly correlates with Renal-SLEDAI and the PGA, and negatively correlates with C3 and C4. Urine ALCAM at week 0 significantly correlates with renal-SLEDAI and the PGA, a scoring system used to assess disease severity, and negatively correlates with the serum complement proteins C3 and C4.
Urine ALCAM significantly correlates with Renal-SLEDAI and the PGA, and negatively correlates with C3 and C4. Urine ALCAM at week 0 significantly correlates with renal-SLEDAI and the PGA, a scoring system used to assess disease severity, and negatively correlates with the serum complement proteins C3 and C4.
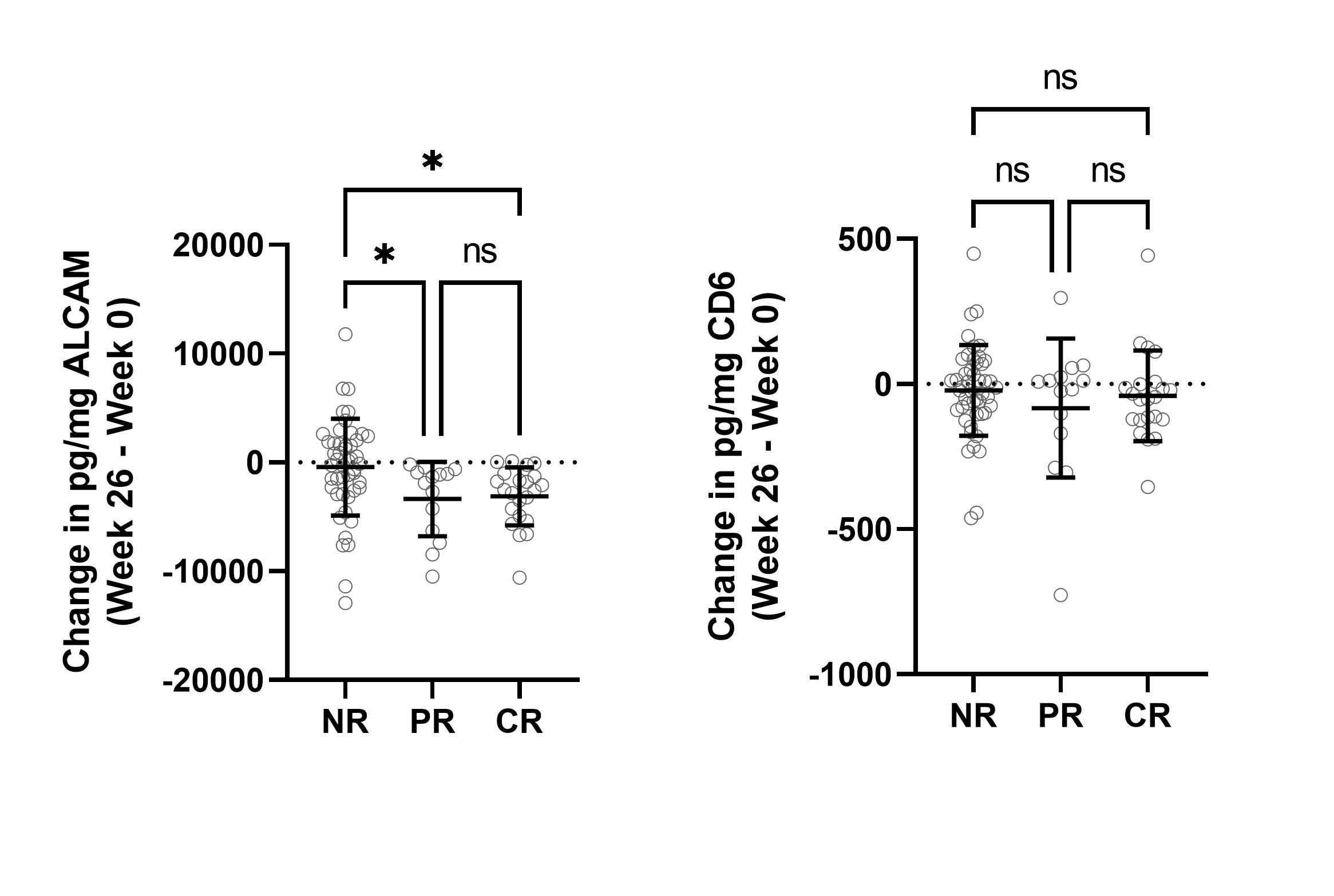 Partial and complete responders experience a significant decrease in urine ALCAM, but not CD6. Change in urine ALCAM and CD6 was determined between week 0 and week 26, and stratified based on response status as determined by AMP. Significant reductions in urine ALCAM are observed for PR and CR.
Partial and complete responders experience a significant decrease in urine ALCAM, but not CD6. Change in urine ALCAM and CD6 was determined between week 0 and week 26, and stratified based on response status as determined by AMP. Significant reductions in urine ALCAM are observed for PR and CR.
Disclosures: D. Chu, Equillium, Inc.; N. Schwartz, None; J. Ampudia, Equillium, Inc.; J. Guthridge, None; J. James, Bristol-Myers Squibb(BMS), AstraZeneca, Novartis, Progentec Biosciences; J. Buyon, None; S. Connelly, Equillium, Inc.; M. Fung, Equillium, Inc.; C. Ng, Equillium, Inc.; A. consortium, None; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; C. Mohan, None; C. Putterman, Equillium, Progentec, Kidneycure.
Background/Purpose: T cells play a critical role in the pathogenicity of SLE and lupus nephritis (LN). Hence, identifying T cell co-stimulatory pathways and mediators that correlate with disease activity may significantly aid in the development of biomarkers applicable for clinical use, and help identify novel therapeutic targets. We found that expression of CD6 (on T cells) and ALCAM (on antigen presenting cells and on tubular cells) is upregulated in the kidneys in LN mouse models and that blockade of the pathway using an anti-CD6 monoclonal antibody significantly reduced the severity of kidney involvement, implicating an important role for the CD6-ALCAM pathway in the development of LN. Furthermore, we found that soluble urine ALCAM is significantly elevated in patients with LN. Here, we expand upon the previous analyses by evaluating urine ALCAM as a predictor of nephritis progression and/or resolution.
Methods: Patient urine and serum samples were acquired through the Accelerating Medicines Partnership (AMP), a public-private partnership to accelerate discovery and development of therapeutics for autoimmune diseases. Samples were obtained from patients with biopsy proven LN and living kidney donor controls and longitudinal sampling (0, 3, 6, and 12 months) was available for 143 LN patients. Soluble levels of ALCAM were quantitated by ELISA, CD6 levels by a proprietary ECL assay, and urine creatinine from the same urine sample was measured by an improved Jaffe method. Urine ALCAM or CD6 levels were then normalized to urine creatinine levels on a pg (ALCAM or CD6) per mg creatinine basis. Responder status (complete response (CR), partial response (PR), no response (NR)) was as determined by AMP. In a separate analysis, responder and non-responder status was determined by change in UPCR where responders showed a decrease in UPCR of greater than 50% from the selected starting point.
Results: Cross-sectional analysis at baseline/week 0 showed that urine ALCAM significantly correlated with renal-SLEDAI (r(250) = 0.2460, p< 0.0001), the Physician Global Assessment (PGA) score (r(198) = 0.3325, p< 0.0001), and negatively with serum C3 (r(255) = -0.2170, p< 0.001) and C4 (r(253) = -0.1368, p< 0.05) (Figure 1). A receiver operating characteristic (ROC) curve was used to assess the sensitivity and specificity of urine ALCAM and CD6 as predictors of LN compared to healthy controls. Urine ALCAM is a strong predictor of active LN with an area under the curve (AUC) of 0.9781, compared to urine CD6 with an AUC of 0.7168. To evaluate longitudinal changes in urine ALCAM or CD6 and their associations with response status, the change between week 0 and week 26 was determined and related back to response status. Partial and complete responders experienced a significant reduction in urine ALCAM compared to non-responders, irrespective of the type of treatment, while urine CD6 did not distinguish between these groups (Figure 2).
Conclusion: Urinary ALCAM is a biomarker of disease severity in LN that could be indicative of a patient's response to treatment. Analyses are in progress to evaluate ALCAM in combination with other measures of disease severity to predict longer term disease outcomes.
 Urine ALCAM significantly correlates with Renal-SLEDAI and the PGA, and negatively correlates with C3 and C4. Urine ALCAM at week 0 significantly correlates with renal-SLEDAI and the PGA, a scoring system used to assess disease severity, and negatively correlates with the serum complement proteins C3 and C4.
Urine ALCAM significantly correlates with Renal-SLEDAI and the PGA, and negatively correlates with C3 and C4. Urine ALCAM at week 0 significantly correlates with renal-SLEDAI and the PGA, a scoring system used to assess disease severity, and negatively correlates with the serum complement proteins C3 and C4.  Partial and complete responders experience a significant decrease in urine ALCAM, but not CD6. Change in urine ALCAM and CD6 was determined between week 0 and week 26, and stratified based on response status as determined by AMP. Significant reductions in urine ALCAM are observed for PR and CR.
Partial and complete responders experience a significant decrease in urine ALCAM, but not CD6. Change in urine ALCAM and CD6 was determined between week 0 and week 26, and stratified based on response status as determined by AMP. Significant reductions in urine ALCAM are observed for PR and CR. Disclosures: D. Chu, Equillium, Inc.; N. Schwartz, None; J. Ampudia, Equillium, Inc.; J. Guthridge, None; J. James, Bristol-Myers Squibb(BMS), AstraZeneca, Novartis, Progentec Biosciences; J. Buyon, None; S. Connelly, Equillium, Inc.; M. Fung, Equillium, Inc.; C. Ng, Equillium, Inc.; A. consortium, None; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; C. Mohan, None; C. Putterman, Equillium, Progentec, Kidneycure.

