Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0423: Direct and Indirect Effect of TNFi on BASMI Components in People with Axial Spondyloarthritis: A Longitudinal Study
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AP
Ana Sofia Pinto, MD
Local Health Unit of Guarda, Portugal
Guarda, Portugal
Abstract Poster Presenter(s)
Ana Pinto1, Claire Harris2, Rhys Hayward2, Andrew Keat2 and Pedro Machado2, 1Local Health Unit of Guarda, Barcelos, Portugal, 2Department of Rheumatology, Northwick Park Hospital, London North West University Healthcare NHS Trust, London, United Kingdom
Background/Purpose: The Bath Ankylosing Spondylitis Metrology Index (BASMI) is an index of spinal mobility for people with axial spondyloarthritis (axSpA). BASMI is one of the few objective measures of spinal disease progression that does not involve imaging. The assessment of mobility and physical function are essential components of the management of axSpA patients and, with the use of early therapeutics that are effective on improving/preserving function, mobility and, potentially structural damage, understanding the interplay between treatments and the various health outcomes affected by axSpA will contribute to better patient management. Our goal was to describe the long-term effect of TNF inhibitors (TNFi) on spinal mobility (each component of BASMI) in patients with axSpA, and to determine whether the use of TNFi treatment influences spinal mobility and if this is due to a direct or indirect effect (mediated by disease activity).
Methods: We performed a longitudinal study, using data routinely collected from patients with a clinical diagnosis of axSpA treated with TNFi at a specialist tertiary care center. Patients with at least two BASMI measurements (one before and one after TNFi initiation) were included. The relationship between TNFi treatment and BASMI over time was investigated using binomial generalized estimating equations (GEE). GEE is a technique that makes use of all available longitudinal data, allows unequal numbers of repeated measurements, corrects for within-subject correlation, and has some robustness against deviation from normality. We built 5 multivariable models; in the first three we investigated the isolated effect of TNFi (model 1), ASDAS (model 2) and BASDAI+CRP (model 3) on spinal mobility (each BASMI component); then we built two additional models, adjusting simultaneously for TNFi treatment and disease activity - one adjusted for TNFi and ASDAS (model 4) and one adjusted for TNFi and BASDAI+CRP (model 5). Other demographic and clinical variables were included as covariates in all multivariable models, including the time period between starting TNFi and the spinal mobility assessment.
Results: Data from 188 patients and 1326 visits were analysed. Mean age was 45.6 (SD 11.6) years, mean disease duration was 15.8 (SD 9.64) years, 152 (80.9%) were male, 120 (73.6%) had radiographic axSpA, and 83 (74.8%) were HLA-B27 positive. Mean follow-up time was 8.0 (SD 4.4) years, ranging from 0.8 to 18.2 years. In the first three models, we observed a significant effect of TNFi in all the BASMI components, as we did for ASDAS and BASDAI (the only exception being for BASDAI, in the tragus-wall distance model). In the models combining TNFi treatment and disease activity, we found that TNFi treatment was significantly associated with improvement in the majority of BASMI components, even after controlling for disease activity (Table 1).
Conclusion: TNFi has a long-term beneficial effect on all BASMI components, which seems to be both due to an indirect effect (mediated by disease activity) and a direct effect of TNFi treatment.
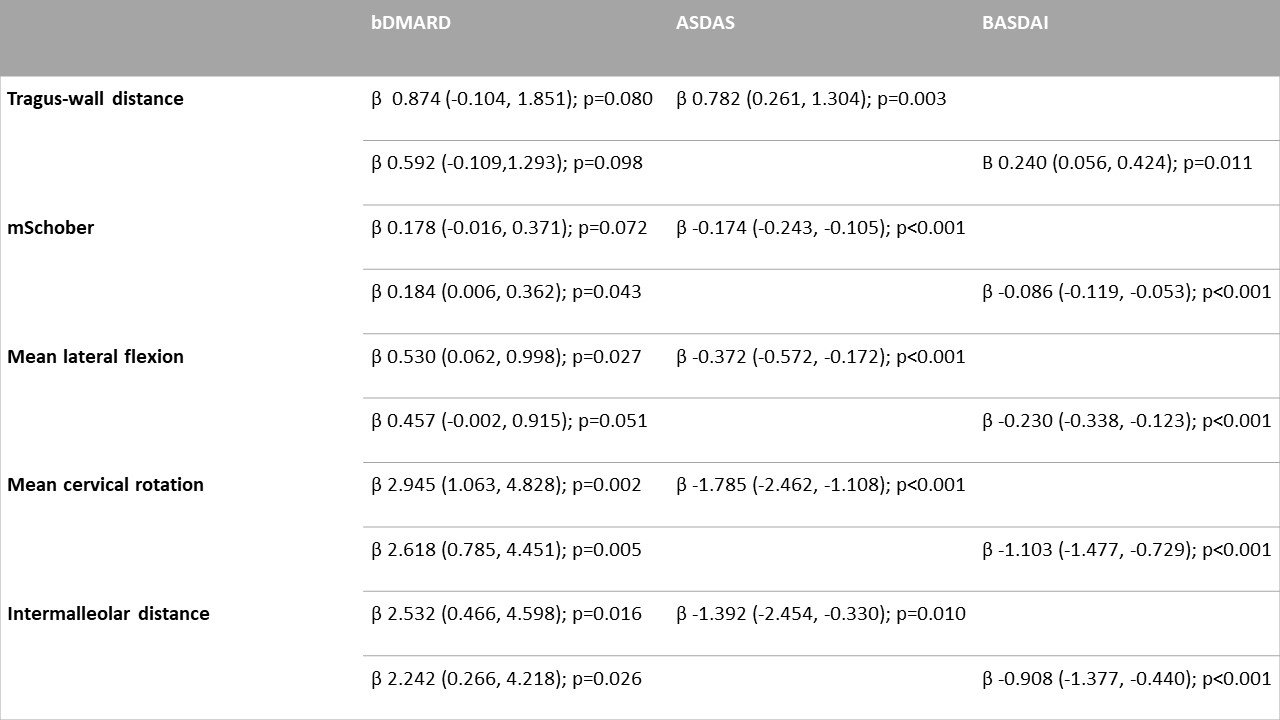 Table 1 - GEE analysis of BASMI items, adjusted for age, gender, bDMARD use, bDMARD duration, Peripheral arthritis, dactylitis, enthesitis, psoriasis, inflammatory bowel disease, uveitis, radiographic axial spondyloarthritis, non-steroid anti-inflammatory drug intake, HLA B27 positivity, BASDAI + CRP or ASDAS
Table 1 - GEE analysis of BASMI items, adjusted for age, gender, bDMARD use, bDMARD duration, Peripheral arthritis, dactylitis, enthesitis, psoriasis, inflammatory bowel disease, uveitis, radiographic axial spondyloarthritis, non-steroid anti-inflammatory drug intake, HLA B27 positivity, BASDAI + CRP or ASDAS
Disclosures: A. Pinto, None; C. Harris, None; R. Hayward, None; A. Keat, None; P. Machado, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Galapagos, Janssen, Merck/MSD, Orphazyme, Novartis, Pfizer, UCB, Roche.
Background/Purpose: The Bath Ankylosing Spondylitis Metrology Index (BASMI) is an index of spinal mobility for people with axial spondyloarthritis (axSpA). BASMI is one of the few objective measures of spinal disease progression that does not involve imaging. The assessment of mobility and physical function are essential components of the management of axSpA patients and, with the use of early therapeutics that are effective on improving/preserving function, mobility and, potentially structural damage, understanding the interplay between treatments and the various health outcomes affected by axSpA will contribute to better patient management. Our goal was to describe the long-term effect of TNF inhibitors (TNFi) on spinal mobility (each component of BASMI) in patients with axSpA, and to determine whether the use of TNFi treatment influences spinal mobility and if this is due to a direct or indirect effect (mediated by disease activity).
Methods: We performed a longitudinal study, using data routinely collected from patients with a clinical diagnosis of axSpA treated with TNFi at a specialist tertiary care center. Patients with at least two BASMI measurements (one before and one after TNFi initiation) were included. The relationship between TNFi treatment and BASMI over time was investigated using binomial generalized estimating equations (GEE). GEE is a technique that makes use of all available longitudinal data, allows unequal numbers of repeated measurements, corrects for within-subject correlation, and has some robustness against deviation from normality. We built 5 multivariable models; in the first three we investigated the isolated effect of TNFi (model 1), ASDAS (model 2) and BASDAI+CRP (model 3) on spinal mobility (each BASMI component); then we built two additional models, adjusting simultaneously for TNFi treatment and disease activity - one adjusted for TNFi and ASDAS (model 4) and one adjusted for TNFi and BASDAI+CRP (model 5). Other demographic and clinical variables were included as covariates in all multivariable models, including the time period between starting TNFi and the spinal mobility assessment.
Results: Data from 188 patients and 1326 visits were analysed. Mean age was 45.6 (SD 11.6) years, mean disease duration was 15.8 (SD 9.64) years, 152 (80.9%) were male, 120 (73.6%) had radiographic axSpA, and 83 (74.8%) were HLA-B27 positive. Mean follow-up time was 8.0 (SD 4.4) years, ranging from 0.8 to 18.2 years. In the first three models, we observed a significant effect of TNFi in all the BASMI components, as we did for ASDAS and BASDAI (the only exception being for BASDAI, in the tragus-wall distance model). In the models combining TNFi treatment and disease activity, we found that TNFi treatment was significantly associated with improvement in the majority of BASMI components, even after controlling for disease activity (Table 1).
Conclusion: TNFi has a long-term beneficial effect on all BASMI components, which seems to be both due to an indirect effect (mediated by disease activity) and a direct effect of TNFi treatment.
 Table 1 - GEE analysis of BASMI items, adjusted for age, gender, bDMARD use, bDMARD duration, Peripheral arthritis, dactylitis, enthesitis, psoriasis, inflammatory bowel disease, uveitis, radiographic axial spondyloarthritis, non-steroid anti-inflammatory drug intake, HLA B27 positivity, BASDAI + CRP or ASDAS
Table 1 - GEE analysis of BASMI items, adjusted for age, gender, bDMARD use, bDMARD duration, Peripheral arthritis, dactylitis, enthesitis, psoriasis, inflammatory bowel disease, uveitis, radiographic axial spondyloarthritis, non-steroid anti-inflammatory drug intake, HLA B27 positivity, BASDAI + CRP or ASDASDisclosures: A. Pinto, None; C. Harris, None; R. Hayward, None; A. Keat, None; P. Machado, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Galapagos, Janssen, Merck/MSD, Orphazyme, Novartis, Pfizer, UCB, Roche.

