Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0413: Efficacy and Safety of Non-Pharmacological and Non-Biological Therapy: A Systematic Literature Review Informing the 2022 Update of the ASAS-EULAR Recommendations for the Management of Axial Spondyloarthritis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Augusta Ortolan, MD
University of Padova
Padova, Padua, Italy
Abstract Poster Presenter(s)
Augusta Ortolan1, Casper Webers2, Alexandre Sepriano3, Louise Falzon4, Xenofon Baraliakos5, Robert Landewé6, Sofia Ramiro7, Désirée van der Heijde8 and Elena Nikiphorou9, 1University of Padova/Leiden University Medical Center, Padova, Italy, 2Maastricht University Medical Centre, Maastricht, Netherlands, 3Leiden University Medical Centre, Portela Loures, Portugal, 4Health Economics and Decision Science, School of Health and Related Research, University of Sheffield, Sheffield, United Kingdom, 5Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 6Amsterdam University Medical Center, Meerssen, Netherlands, 7Leiden University Medical Center, Leiden, Netherlands, 8Department of Rheumatology, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Netherlands, 9Leiden University Medical Center & King's College London, London, United Kingdom
Background/Purpose: Since the 2016 recommendations for axial spondyloarthritis (axSpA) management, new evidence emerged regarding axSpA treatment. The aim of this work was to update the evidence on efficacy and safety for non-pharmacological and non-biological treatments to inform the update of ASAS-EULAR recommendations for the management of axSpA.
Methods: Systematic literature review (SLR) from 2016 (previous SLR), up to 1st January 2022 (PROSPERO registration CRD42021261959). The research question was formulated according to the PICO format: Population: adult patients with r-axSpA or nr-axSpA; Intervention: non-pharmacological and non-biological treatments; Comparator: active comparator or placebo; Outcomes: all relevant efficacy and safety outcomes; Type of studies: randomised controlled trials (RCTs) and observational studies (the latter for efficacy of non-pharmacological treatments and safety only). Cohen's effect size (ES) was calculated for non-pharmacological and risk ratio (RR) for pharmacological treatments. Risk of bias (RoB) was assessed using the Cochrane tool for RCTs and the Hayden tool for cohort studies.
Results: After title/abstract screening and full-text reading, 117 publications were included in the present SLR. Studies on non-pharmacological interventions addressed education (n=8), exercise (n=20) and other treatments (n=16). The majority of these studies were conducted on r-axSpA, and were with high RoB, except 6 RCTs at unclear RoB. The ES for education on disease activity, function, and mobility were small to moderate (ES min/max for BASDAI: -1.2/0.59, BASFI: -0.25/0.58, BASMI: 0.07/0.54). The ES for exercise regarding the same outcomes were moderate to high (ES min/max for ASDAS: 0.29/0.94, BASDAI: 0.14/1.43, BASFI: 0.04/0.92, BASMI: 0.06/1.14). Studies describing other types of non-pharmacological interventions (e.g. ultrasound therapy, moxibustion, diet) were very heterogeneous in the type, duration, and frequency of the treatments. Studies on conventional synthetic DMARDs (n=3), NSAIDs (n=8) and other drugs (n=12) did not provide new relevant evidence on efficacy or safety. Among 12 studies addressing other pharmacological interventions, one at unclear RoB showed limited efficacy of short-term glucocorticoids in axSpA (ASAS20 RR 1.80; 95%CI: 0.87-3.70; ASAS40 RR 2.47, 95%CI 0.98-6.22). One RCT at unclear RoB has shown that alendronate is not efficacious in r-axSpA. Six RCTs on 5 targeted synthetic DMARDs (tsDMARDs) showed efficacy of tofacitinib, upadacitinib and filgotinib, only in r-axSpA (Table). Two RCTs showed that apremilast and nilotinib are not efficacious in r-axSpA (BASDAI 50 more often reached in placebo -33%- than nilotinib -0%).
Conclusion: New evidence on education and exercise confirms their efficacy in axSpA. Within the new class of tsDMARDs tofacitinib, upadacitinib and filgotinib were those which have shown efficacy in r-axSpA.
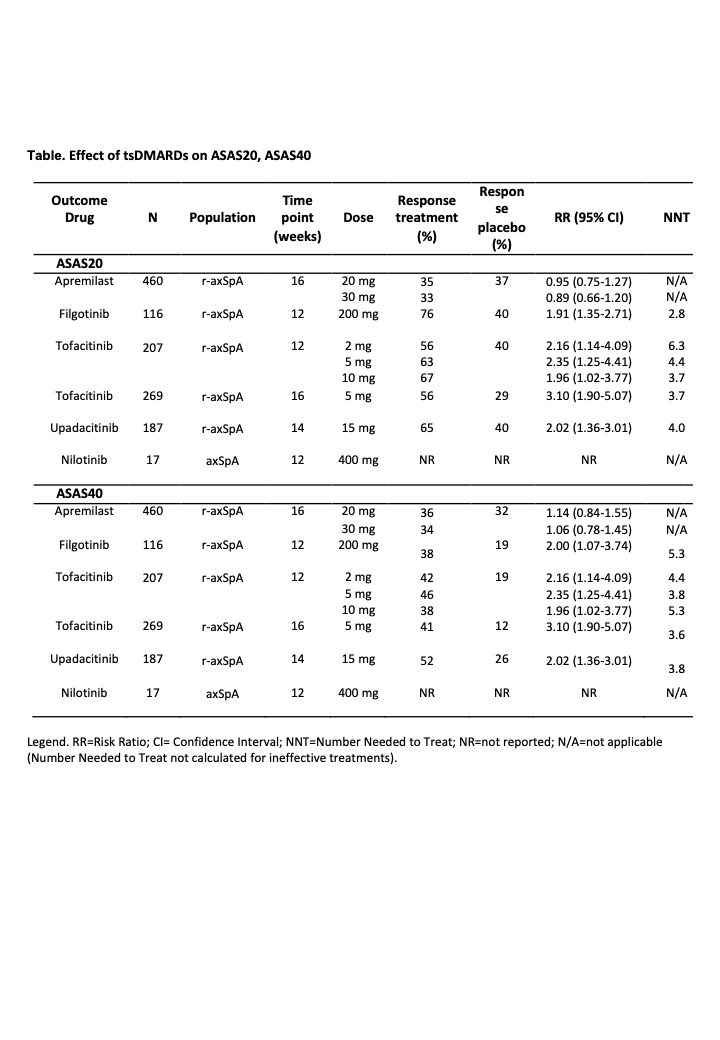 Table. Effect of tsDMARDs on ASAS20 and ASAS40
Table. Effect of tsDMARDs on ASAS20 and ASAS40
Disclosures: A. Ortolan, None; C. Webers, None; A. Sepriano, UCB, Novartis; L. Falzon, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; S. Ramiro, AbbVie/Abbott, Eli Lilly, Galapagos, Merck/MSD, Novartis, Pfizer, UCB, Sanofi; D. van der Heijde, AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Novartis, Pfizer, UCB, Imaging Rheumatology bv, Lilly; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius.
Background/Purpose: Since the 2016 recommendations for axial spondyloarthritis (axSpA) management, new evidence emerged regarding axSpA treatment. The aim of this work was to update the evidence on efficacy and safety for non-pharmacological and non-biological treatments to inform the update of ASAS-EULAR recommendations for the management of axSpA.
Methods: Systematic literature review (SLR) from 2016 (previous SLR), up to 1st January 2022 (PROSPERO registration CRD42021261959). The research question was formulated according to the PICO format: Population: adult patients with r-axSpA or nr-axSpA; Intervention: non-pharmacological and non-biological treatments; Comparator: active comparator or placebo; Outcomes: all relevant efficacy and safety outcomes; Type of studies: randomised controlled trials (RCTs) and observational studies (the latter for efficacy of non-pharmacological treatments and safety only). Cohen's effect size (ES) was calculated for non-pharmacological and risk ratio (RR) for pharmacological treatments. Risk of bias (RoB) was assessed using the Cochrane tool for RCTs and the Hayden tool for cohort studies.
Results: After title/abstract screening and full-text reading, 117 publications were included in the present SLR. Studies on non-pharmacological interventions addressed education (n=8), exercise (n=20) and other treatments (n=16). The majority of these studies were conducted on r-axSpA, and were with high RoB, except 6 RCTs at unclear RoB. The ES for education on disease activity, function, and mobility were small to moderate (ES min/max for BASDAI: -1.2/0.59, BASFI: -0.25/0.58, BASMI: 0.07/0.54). The ES for exercise regarding the same outcomes were moderate to high (ES min/max for ASDAS: 0.29/0.94, BASDAI: 0.14/1.43, BASFI: 0.04/0.92, BASMI: 0.06/1.14). Studies describing other types of non-pharmacological interventions (e.g. ultrasound therapy, moxibustion, diet) were very heterogeneous in the type, duration, and frequency of the treatments. Studies on conventional synthetic DMARDs (n=3), NSAIDs (n=8) and other drugs (n=12) did not provide new relevant evidence on efficacy or safety. Among 12 studies addressing other pharmacological interventions, one at unclear RoB showed limited efficacy of short-term glucocorticoids in axSpA (ASAS20 RR 1.80; 95%CI: 0.87-3.70; ASAS40 RR 2.47, 95%CI 0.98-6.22). One RCT at unclear RoB has shown that alendronate is not efficacious in r-axSpA. Six RCTs on 5 targeted synthetic DMARDs (tsDMARDs) showed efficacy of tofacitinib, upadacitinib and filgotinib, only in r-axSpA (Table). Two RCTs showed that apremilast and nilotinib are not efficacious in r-axSpA (BASDAI 50 more often reached in placebo -33%- than nilotinib -0%).
Conclusion: New evidence on education and exercise confirms their efficacy in axSpA. Within the new class of tsDMARDs tofacitinib, upadacitinib and filgotinib were those which have shown efficacy in r-axSpA.
 Table. Effect of tsDMARDs on ASAS20 and ASAS40
Table. Effect of tsDMARDs on ASAS20 and ASAS40Disclosures: A. Ortolan, None; C. Webers, None; A. Sepriano, UCB, Novartis; L. Falzon, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; S. Ramiro, AbbVie/Abbott, Eli Lilly, Galapagos, Merck/MSD, Novartis, Pfizer, UCB, Sanofi; D. van der Heijde, AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Novartis, Pfizer, UCB, Imaging Rheumatology bv, Lilly; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius.

