Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0422: Efficacy and Safety of Secukinumab in the Treatment of Axial Spondyloarthritis: Real-Life Data from TURKBIO Cohort
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SG
Semih Gülle, MD
Dokuz Eylul University School of Medicine
Izmir, Turkey
Abstract Poster Presenter(s)
Semih Gulle1, Ali Karakas1, Gercek Can2, Soner Senel3, Sedat Capar4, Huseyin ediz dalkilic5, Servet Akar6, Suleyman Serdar Koca7, Abdurrahman Tufan8, Ayten Yazici9, Sema Yilmaz10, Nevsun Inanc11, Merih Birlik1, Dilek Solmaz12, Ayse Cefle9, Berna Goker13, Servet Yolbas14, Niels Steen Krough15, Neslihan Yilmaz16, Sukran Erten17, Cemal Bes18, Ozgul Soysal19, Mehmet Akif Ozturk13, Seminur Haznedaroglu13, Sule Yavuz16, Haner Direskeneli20, Fatoş Onen21 and Ismail Sari22, 1Dokuz Eylul University School of Medicine Division of Rheumatology, Izmir, Turkey, 2Dokuz Eylul University School of Medicine Division of Rheumatology, Istanbul, Turkey, 3Erciyes University School of Medicine Division of Rheumatology, Kayseri, Turkey, 4Dokuz Eylul University Faculty of Science Department of Statistics, Izmir, Turkey, 5Uludag University School of Medicine Division of Rheumatology, Bursa, Turkey, 6Izmir Katip Celebi University School of Medicine, Izmir, Turkey, 7Firat University School of Medicine Division of Rheumatology, Elazıg, Turkey, 8Gazi University Medical Faculty Hospital, Istanbul, Turkey, 9Kocaeli University School of Medicine Division of Rheumatology, Kocaeli, Turkey, 10Selcuk University School of Medicine Division of Rheumatology, Konya, Turkey, 11Marmara University School of Medicine, Division of Rheumatology, Istanbul, Turkey, 12Kâtip Celebi University School of Medicine Division of Rheumatology, Izmir, Turkey, 13Gazi University School of Medicine Division of Rheumatology, Ankara, Turkey, 14Inonu University School of Medicine Division of Rheumatology, Malatya, Turkey, 15Zitelab Aps, Kopenhag, Denmark, 16Demiroglu Bilim University School of Medicine Division of Rheumatology, Istanbul, Turkey, 17Yildirim Beyazit University School of Medicine Division of Rheumatology, Ankara, Turkey, 18Basaksehir Cam and Sakura ospital, Division of Rheumatology, Istanbul, Turkey, 19Celal Bayar University, School of Medicine Division of Rheumatology, Manisa, Turkey, 20Marmara University School of Medicine Division of Rheumatology, Istanbul, Turkey, 21Dokuz Eylul University, Faculty of Medicine, Rheumatology, İzmir, Turkey, 22Dokuz Eylul University School of Medicine Division of Rheumatology, İzmir, Turkey
Background/Purpose: In this study, we aimed to evaluate the results of secukinumab treatment in patients with Axial Spondyloarthritis (AxSpA) who were enrolled in the TURKBIO cohort.
Methods: In our study, AxSpA patients registered in the TUKRBIO database were evaluated. 6, 12 and 24 months of secukinumab drug retention, inactive disease/low disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] < 2/< 4, Ankylosing Spondylitis Disease Activity Score [ASDAS] < 1.3/< 2.1) and response rates (BASDAI50, Spondyloarthritis International Society [ASAS] 20/40, ASDAS clinically significant improvement [ASDAS-CII] and ASDAS major improvement [ASDAS-MI]) were evaluated.
Results: Follow-up data of 184 AS patients registered in the TURKBIO registry were evaluated. Of these patients, 52 (28.3%) were bDMARD naive patients and 132 (71.7) had used at least one bDMARD prior to secukinumab. Age and gender distribution were similar between bDMARD naive and ≥1 bDMARD groups. At 1-year follow-up, 60 and 12 patients discontinued their treatment in the ≥1 bDMARD group and the secukinumab group, respectively (p< 0.001). Ineffectiveness was the most common reason for discontinuing treatment in the ≥1 bDMARD and secukinumab groups. The rate of continuation of secukinumab treatment was 71.7% at 6 months and 60.9% at 12 months. When clinical conditions were assessed based on prior bDMARD use, there were significantly better drug survival rates at 6 months and 12 months for the bDMARD naive group (82.7% and 76.9 months, respectively). In addition, it was determined that the symptoms and the time elapsed after diagnosis did not affect the efficacy of secukinumab. In our study, no significant drug safety problem was encountered in the follow-up of secukinumab treatment for more than 3 years.
Conclusion: In a study conducted with TURKBIO real-life data, we found that secukinumab treatment in patients with AxSpA had better clinical response and higher drug survival rates in biologically naive patients, similar to TNFi treatment. However, it has been observed that the use of secukinumab in the second or subsequent steps in patients who do not respond to biologics has similar success rates to TNFi treatment results.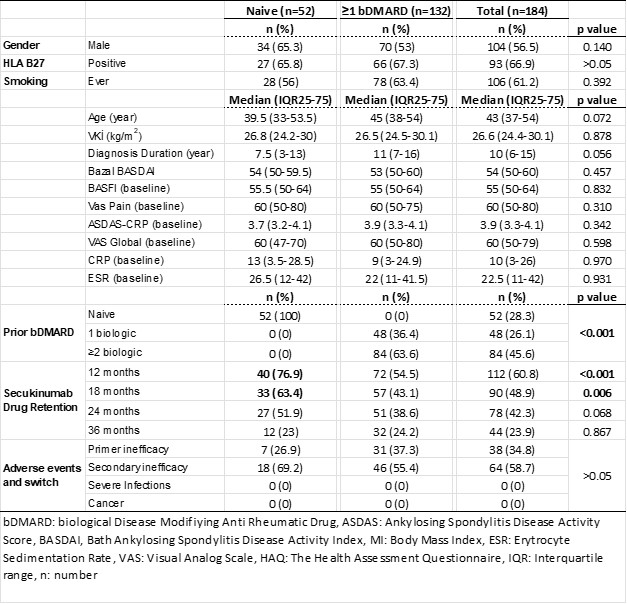 Table 1 Demographic and clinical characteristics of AxSpA patients
Table 1 Demographic and clinical characteristics of AxSpA patients
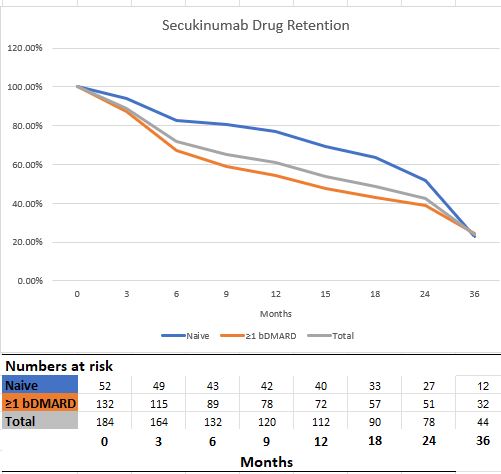 Graphic 1. Secukinumab 3-year drug retention
Graphic 1. Secukinumab 3-year drug retention
Disclosures: S. Gulle, None; A. Karakas, None; G. Can, None; S. Senel, None; S. Capar, None; H. dalkilic, None; S. Akar, AbbVie, Lilly, MSD, Novartis, Pfizer, UCB; S. Koca, None; A. Tufan, None; A. Yazici, None; S. Yilmaz, None; N. Inanc, AbbVie/Abbott, Eli Lilly, Merck/MSD, novartis, Pfizer, Roche, Amgen, Celltrion, UCB; M. Birlik, None; D. Solmaz, None; A. Cefle, None; B. Goker, None; S. Yolbas, None; N. Krough, None; N. Yilmaz, None; S. Erten, None; C. Bes, None; O. Soysal, None; M. Ozturk, None; S. Haznedaroglu, None; S. Yavuz, None; H. Direskeneli, None; F. Onen, None; I. Sari, None.
Background/Purpose: In this study, we aimed to evaluate the results of secukinumab treatment in patients with Axial Spondyloarthritis (AxSpA) who were enrolled in the TURKBIO cohort.
Methods: In our study, AxSpA patients registered in the TUKRBIO database were evaluated. 6, 12 and 24 months of secukinumab drug retention, inactive disease/low disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] < 2/< 4, Ankylosing Spondylitis Disease Activity Score [ASDAS] < 1.3/< 2.1) and response rates (BASDAI50, Spondyloarthritis International Society [ASAS] 20/40, ASDAS clinically significant improvement [ASDAS-CII] and ASDAS major improvement [ASDAS-MI]) were evaluated.
Results: Follow-up data of 184 AS patients registered in the TURKBIO registry were evaluated. Of these patients, 52 (28.3%) were bDMARD naive patients and 132 (71.7) had used at least one bDMARD prior to secukinumab. Age and gender distribution were similar between bDMARD naive and ≥1 bDMARD groups. At 1-year follow-up, 60 and 12 patients discontinued their treatment in the ≥1 bDMARD group and the secukinumab group, respectively (p< 0.001). Ineffectiveness was the most common reason for discontinuing treatment in the ≥1 bDMARD and secukinumab groups. The rate of continuation of secukinumab treatment was 71.7% at 6 months and 60.9% at 12 months. When clinical conditions were assessed based on prior bDMARD use, there were significantly better drug survival rates at 6 months and 12 months for the bDMARD naive group (82.7% and 76.9 months, respectively). In addition, it was determined that the symptoms and the time elapsed after diagnosis did not affect the efficacy of secukinumab. In our study, no significant drug safety problem was encountered in the follow-up of secukinumab treatment for more than 3 years.
Conclusion: In a study conducted with TURKBIO real-life data, we found that secukinumab treatment in patients with AxSpA had better clinical response and higher drug survival rates in biologically naive patients, similar to TNFi treatment. However, it has been observed that the use of secukinumab in the second or subsequent steps in patients who do not respond to biologics has similar success rates to TNFi treatment results.
 Table 1 Demographic and clinical characteristics of AxSpA patients
Table 1 Demographic and clinical characteristics of AxSpA patients  Graphic 1. Secukinumab 3-year drug retention
Graphic 1. Secukinumab 3-year drug retentionDisclosures: S. Gulle, None; A. Karakas, None; G. Can, None; S. Senel, None; S. Capar, None; H. dalkilic, None; S. Akar, AbbVie, Lilly, MSD, Novartis, Pfizer, UCB; S. Koca, None; A. Tufan, None; A. Yazici, None; S. Yilmaz, None; N. Inanc, AbbVie/Abbott, Eli Lilly, Merck/MSD, novartis, Pfizer, Roche, Amgen, Celltrion, UCB; M. Birlik, None; D. Solmaz, None; A. Cefle, None; B. Goker, None; S. Yolbas, None; N. Krough, None; N. Yilmaz, None; S. Erten, None; C. Bes, None; O. Soysal, None; M. Ozturk, None; S. Haznedaroglu, None; S. Yavuz, None; H. Direskeneli, None; F. Onen, None; I. Sari, None.

