Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0299: Radiographic Change in Patients with Rheumatoid Arthritis and Estimated Baseline Yearly Progression ≥5 or < 5: Post Hoc Analysis of Two Phase 3 Trials of Filgotinib
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Yoshiya Tanaka, MD, PhD
University of Occupational and Environmental Health, Japan
Kitakyushu, Japan
Abstract Poster Presenter(s)
Yoshiya Tanaka1, Tatsuya Atsumi2, Daniel Aletaha3, Robert Landewé4, Beatrix Bartok5, Alena Pechonkina6, Ling Han6, Kahaku Emoto7, Shungo Kano7, Vijay Rajendran8 and Tsutomu Takeuchi9, 1University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 2Hokkaido University, Sapporo, Japan, 3Medical University Vienna, Wien, Austria, 4Amsterdam University Medical Center, Meerssen, Netherlands, 5Gilead Sciences, Inc., La Jolla, CA, 6Gilead Sciences, Foster City, CA, 7Gilead Sciences, K.K., Tokyo, Japan, 8Galapagos NV, Gent, Belgium, 9Keio University and Saitama Medical University, Tokyo, Japan
Background/Purpose: In some patients (pts) with rheumatoid arthritis (RA), especially those with joint damage early in the disease, first-line methotrexate (MTX) treatment may not suffice to prevent further rapid radiographic progression (RRP).1 In FINCH 1 (NCT02889796), filgotinib 200 mg (FIL200) and 100 mg (FIL100) reduced change in modified total Sharp score (mTSS) vs placebo (PBO) in pts with RA and inadequate response to MTX (MTX-IR).2 In FINCH 3 (NCT02886728), FIL200 and FIL100 reduced change in mTSS vs MTX monotherapy (MTX mono) in MTX-naïve pts.3
This study was conducted to evaluate, via post hoc analysis of 2 trials, filgotinib's effects on radiographic progression vs MTX mono in pts with estimated baseline (BL) yearly progression ≥5 or < 5 mTSS units/year.
Methods: The double-blind 52-week (W) FINCH 1 study randomized MTX-IR pts with moderate–severe active RA to FIL200 or FIL100, subcutaneous adalimumab (ADA) 40 mg, or PBO; at W24, PBO pts were rerandomized blinded to FIL200 or FIL100; all took stable background MTX.2 In FINCH 3, MTX-naïve pts were randomized, blinded, to FIL200 + MTX, FIL100 + MTX, FIL200 alone, or MTX mono for up to W52.3 This analysis examined subgroups by estimated BL yearly progression (BL mTSS/duration in years of RA diagnosis), based on ≥5 or < 5 mTSS units/year,4 a threshold commonly used to define RRP. We assessed effects of filgotinib vs ADA or PBO in mTSS change from BL (CFB) at W24/W52 (using a mixed model for repeated measures) and percentages with no progression (mTSS change ≤0, ≤0.5, ≤smallest detectable change [SDC], using Fisher's exact test).
Results: At BL, 558/1755 MTX-IR and 787/1249 MTX-naïve pts had BL estimated yearly progression ≥5. Median mTSS in pts with BL yearly progression ≥5 and < 5 was 53.25 vs 5.00 respectively in the MTX-IR trial and 6.00 vs 2.50 in the MTX-naïve trial. At W24, the mTSS CFB in pts with BL yearly progression ≥5 and < 5 was 0.84 and 0.22 in MTX-IR pts taking PBO + MTX, and 0.67 and 0.25 in MTX-naïve pts taking MTX mono. At W52, in pts with BL yearly progression ≥5, FIL200 + MTX reduced mTSS change vs PBO + MTX in the MTX-IR trial and vs MTX mono in the MTX-naïve trial (Figure 1). At W24, among pts with estimated BL yearly progression ≥5, FIL200 + MTX increased odds of no progression (≤0.5 or ≤0) vs PBO + MTX in MTX-IR pts and vs MTX mono in MTX-naïve pts (Table 1).
Conclusion: These data suggest filgotinib's inhibition of radiographic progression was numerically greater than MTX monotherapy in RA pts with high estimated BL yearly progression. In those with a more moderate estimated rate of progression, filgotinib suppressed progression comparably to ADA and/or MTX.
References: 1. Smolen JS et al. Ann Rheum Dis. 2018;77:1566–1572. 2. Combe B et al. Ann Rheum Dis. 2021;80:848–858. 3. Westhovens R et al. Ann Rheum. Dis 2021;80:727–738. 4. Vastesaeger N et al. Rheumatology. 2009;48:1114–1121.
.jpg) Figure 1. CFB in mTSS at W24 and W52. W52 results include data from Campaign A (all radiographs through W24) and Campaign B (radiographs through W52 including re-reading of BL and W24 radiographs). ADA, adalimumab; BL, baseline; CFB, change from baseline; FIL100, filgotinib 100 mg; FIL200, filgotinib 200 mg; IR, inadequate response; LS, least squares; mono, monotherapy; mTSS, modified total Sharp score; MTX, methotrexate; PBO, placebo; W, week.
Figure 1. CFB in mTSS at W24 and W52. W52 results include data from Campaign A (all radiographs through W24) and Campaign B (radiographs through W52 including re-reading of BL and W24 radiographs). ADA, adalimumab; BL, baseline; CFB, change from baseline; FIL100, filgotinib 100 mg; FIL200, filgotinib 200 mg; IR, inadequate response; LS, least squares; mono, monotherapy; mTSS, modified total Sharp score; MTX, methotrexate; PBO, placebo; W, week.
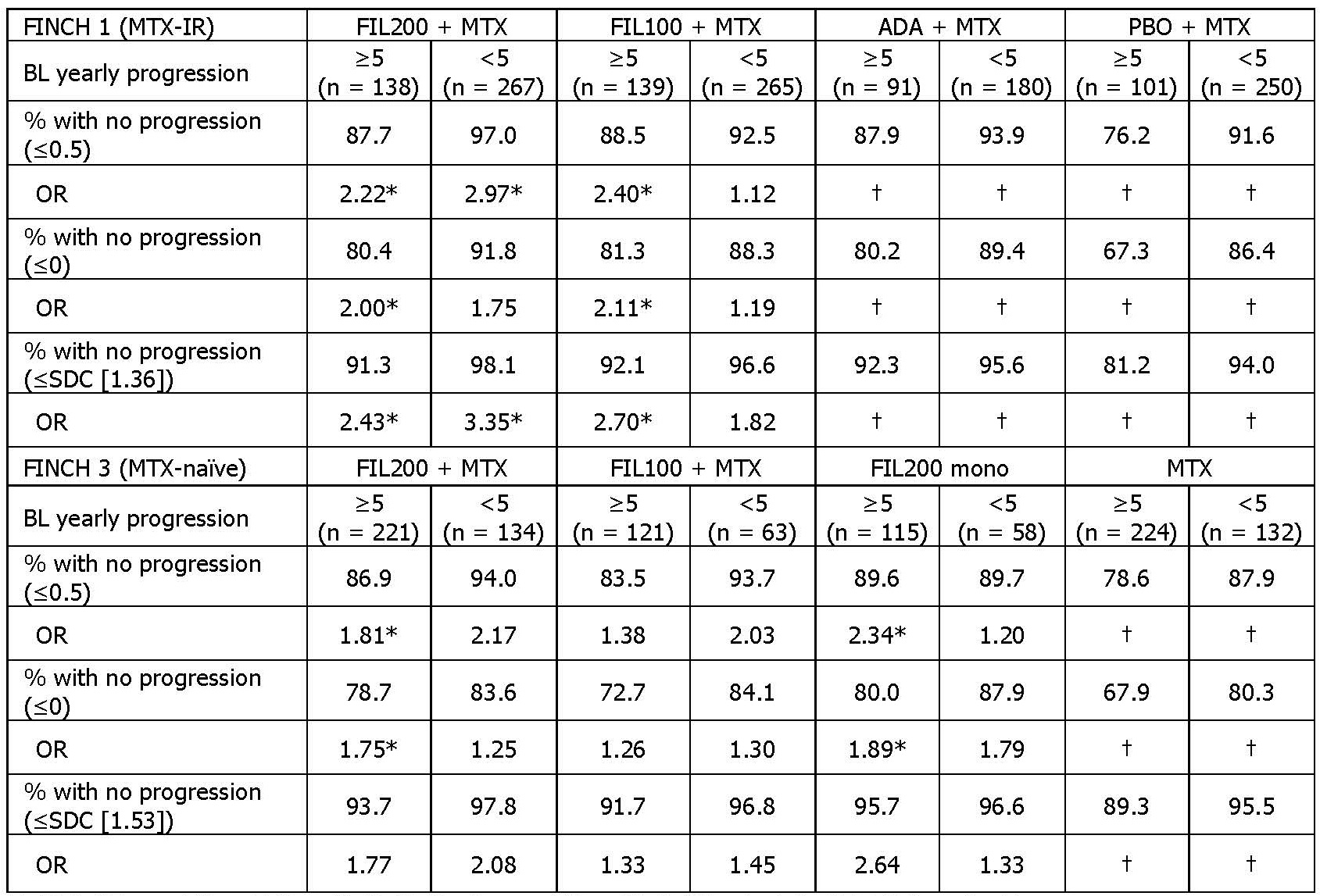 Table 1. Ratio of no radiographic progression at W24. MTX-IR ORs are FIL vs PBO + MTX; MTX-naïve are FIL vs MTX. *Nominal P < .05. †Not applicable. ADA, adalimumab; FIL, filgotinib; IR, inadequate response; mTSS, modified total Sharp score; MTX, methotrexate; OR, odds ratio; SDC, smallest detectable change; W, week.
Table 1. Ratio of no radiographic progression at W24. MTX-IR ORs are FIL vs PBO + MTX; MTX-naïve are FIL vs MTX. *Nominal P < .05. †Not applicable. ADA, adalimumab; FIL, filgotinib; IR, inadequate response; mTSS, modified total Sharp score; MTX, methotrexate; OR, odds ratio; SDC, smallest detectable change; W, week.
Disclosures: Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; T. Atsumi, Alexion, Chugai Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer, Teijin Pharma, AbbVie, Bristol-Myers Squibb (BMS), Eisai, Eli Lilly Japan, Kyowa Kirin, Novartis, Taiho Pharmaceutical, Takeda Pharmaceutical, UCB Pharma, AstraZeneca, Medical and Biological Laboratories, Boehringer-Ingelheim, Ono Pharmaceutical, Gilead Sciences, Inc., Astellas Pharma; D. Aletaha, Novartis, SoBi, Sanofi, Amgen, Lilly, Merck, Pfizer, Roche, Sandoz, Janssen, AbbVie; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; B. Bartok, Gilead Sciences, Inc.; A. Pechonkina, Gilead Sciences, Inc.; L. Han, Gilead Sciences, Inc.; K. Emoto, Gilead Sciences, K.K.; S. Kano, Gilead Sciences, K.K.; V. Rajendran, Galapagos; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi.
Background/Purpose: In some patients (pts) with rheumatoid arthritis (RA), especially those with joint damage early in the disease, first-line methotrexate (MTX) treatment may not suffice to prevent further rapid radiographic progression (RRP).1 In FINCH 1 (NCT02889796), filgotinib 200 mg (FIL200) and 100 mg (FIL100) reduced change in modified total Sharp score (mTSS) vs placebo (PBO) in pts with RA and inadequate response to MTX (MTX-IR).2 In FINCH 3 (NCT02886728), FIL200 and FIL100 reduced change in mTSS vs MTX monotherapy (MTX mono) in MTX-naïve pts.3
This study was conducted to evaluate, via post hoc analysis of 2 trials, filgotinib's effects on radiographic progression vs MTX mono in pts with estimated baseline (BL) yearly progression ≥5 or < 5 mTSS units/year.
Methods: The double-blind 52-week (W) FINCH 1 study randomized MTX-IR pts with moderate–severe active RA to FIL200 or FIL100, subcutaneous adalimumab (ADA) 40 mg, or PBO; at W24, PBO pts were rerandomized blinded to FIL200 or FIL100; all took stable background MTX.2 In FINCH 3, MTX-naïve pts were randomized, blinded, to FIL200 + MTX, FIL100 + MTX, FIL200 alone, or MTX mono for up to W52.3 This analysis examined subgroups by estimated BL yearly progression (BL mTSS/duration in years of RA diagnosis), based on ≥5 or < 5 mTSS units/year,4 a threshold commonly used to define RRP. We assessed effects of filgotinib vs ADA or PBO in mTSS change from BL (CFB) at W24/W52 (using a mixed model for repeated measures) and percentages with no progression (mTSS change ≤0, ≤0.5, ≤smallest detectable change [SDC], using Fisher's exact test).
Results: At BL, 558/1755 MTX-IR and 787/1249 MTX-naïve pts had BL estimated yearly progression ≥5. Median mTSS in pts with BL yearly progression ≥5 and < 5 was 53.25 vs 5.00 respectively in the MTX-IR trial and 6.00 vs 2.50 in the MTX-naïve trial. At W24, the mTSS CFB in pts with BL yearly progression ≥5 and < 5 was 0.84 and 0.22 in MTX-IR pts taking PBO + MTX, and 0.67 and 0.25 in MTX-naïve pts taking MTX mono. At W52, in pts with BL yearly progression ≥5, FIL200 + MTX reduced mTSS change vs PBO + MTX in the MTX-IR trial and vs MTX mono in the MTX-naïve trial (Figure 1). At W24, among pts with estimated BL yearly progression ≥5, FIL200 + MTX increased odds of no progression (≤0.5 or ≤0) vs PBO + MTX in MTX-IR pts and vs MTX mono in MTX-naïve pts (Table 1).
Conclusion: These data suggest filgotinib's inhibition of radiographic progression was numerically greater than MTX monotherapy in RA pts with high estimated BL yearly progression. In those with a more moderate estimated rate of progression, filgotinib suppressed progression comparably to ADA and/or MTX.
References: 1. Smolen JS et al. Ann Rheum Dis. 2018;77:1566–1572. 2. Combe B et al. Ann Rheum Dis. 2021;80:848–858. 3. Westhovens R et al. Ann Rheum. Dis 2021;80:727–738. 4. Vastesaeger N et al. Rheumatology. 2009;48:1114–1121.
.jpg) Figure 1. CFB in mTSS at W24 and W52. W52 results include data from Campaign A (all radiographs through W24) and Campaign B (radiographs through W52 including re-reading of BL and W24 radiographs). ADA, adalimumab; BL, baseline; CFB, change from baseline; FIL100, filgotinib 100 mg; FIL200, filgotinib 200 mg; IR, inadequate response; LS, least squares; mono, monotherapy; mTSS, modified total Sharp score; MTX, methotrexate; PBO, placebo; W, week.
Figure 1. CFB in mTSS at W24 and W52. W52 results include data from Campaign A (all radiographs through W24) and Campaign B (radiographs through W52 including re-reading of BL and W24 radiographs). ADA, adalimumab; BL, baseline; CFB, change from baseline; FIL100, filgotinib 100 mg; FIL200, filgotinib 200 mg; IR, inadequate response; LS, least squares; mono, monotherapy; mTSS, modified total Sharp score; MTX, methotrexate; PBO, placebo; W, week. Table 1. Ratio of no radiographic progression at W24. MTX-IR ORs are FIL vs PBO + MTX; MTX-naïve are FIL vs MTX. *Nominal P < .05. †Not applicable. ADA, adalimumab; FIL, filgotinib; IR, inadequate response; mTSS, modified total Sharp score; MTX, methotrexate; OR, odds ratio; SDC, smallest detectable change; W, week.
Table 1. Ratio of no radiographic progression at W24. MTX-IR ORs are FIL vs PBO + MTX; MTX-naïve are FIL vs MTX. *Nominal P < .05. †Not applicable. ADA, adalimumab; FIL, filgotinib; IR, inadequate response; mTSS, modified total Sharp score; MTX, methotrexate; OR, odds ratio; SDC, smallest detectable change; W, week.Disclosures: Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; T. Atsumi, Alexion, Chugai Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer, Teijin Pharma, AbbVie, Bristol-Myers Squibb (BMS), Eisai, Eli Lilly Japan, Kyowa Kirin, Novartis, Taiho Pharmaceutical, Takeda Pharmaceutical, UCB Pharma, AstraZeneca, Medical and Biological Laboratories, Boehringer-Ingelheim, Ono Pharmaceutical, Gilead Sciences, Inc., Astellas Pharma; D. Aletaha, Novartis, SoBi, Sanofi, Amgen, Lilly, Merck, Pfizer, Roche, Sandoz, Janssen, AbbVie; R. Landewé, Abbott, Amgen, AstraZeneca, BMS, GSK, Novartis, Merck, Pfizer, Schering-Plough, UCB Pharma; B. Bartok, Gilead Sciences, Inc.; A. Pechonkina, Gilead Sciences, Inc.; L. Han, Gilead Sciences, Inc.; K. Emoto, Gilead Sciences, K.K.; S. Kano, Gilead Sciences, K.K.; V. Rajendran, Galapagos; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi.

