Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0307: Reduction in Structural Damage Progression and Improvement in Clinical Response with Abatacept Treatment in Patients with ACPA+, Early RA: Results from a Post Hoc Analysis of the AVERT-2 Study by Baseline Erosion Scores
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- CP
Chahin Pachai, PhD
Bristol Myers Squibb
Princeton, NJ, United States
Abstract Poster Presenter(s)
Chahin Pachai1, Sean E Connolly2, Yoshiya Tanaka3, Vivian Bykerk4, Tom Huizinga5, Gustavo Citera6, Clifton O. Bingham III7, Shuyan Du1, Godehard Hoexter8, Roy Fleischmann9, Subhashis Banerjee1 and Paul Emery10, 1Bristol Myers Squibb, Princeton, NJ, 2Bristol-Myers Squibb, Morrisville, PA, 3University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 4Hospital for Special Surgery, New York, NY, 5Leiden University Medical Center, Leiden, Netherlands, 6Instituto de Rehabilitación Psicofísica (IREP), Buenos Aires, Argentina, 7Johns Hopkins University, Baltimore, MD, 8Bristol Myers Squibb GmbH & Co. KGaA, Munich, Germany, 9University of Texas Southwestern Medical Center and Metroplex Clinical Research Center, Dallas, TX, 10Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom
Background/Purpose: Presence of structural damage is an important risk factor for further damage progression over time in RA. Inhibition of damage progression is a key outcome measure in RA trials.
We assessed the effect of baseline (BL) structural damage, based on X-ray erosion scores (ES), on the progression of structural damage and clinical response over 52 weeks (wks) with subcutaneous (SC) abatacept (ABA) treatment in MTX-naïve, ACPA+ patients (pts) with early RA in the phase 3b AVERT-2 study (NCT02504268).1
Methods: Pts with early RA (ACR/EULAR 2010)2 were randomized to weekly SC ABA 125 mg or SC placebo (PBO), both with oral MTX, for 56 wks. X-rays were evaluated using modified total Sharp/van der Heijde score. This retrospective analysis included all pts with X-rays (BL and wk 52) and select clinical outcomes available.
Comparisons were made between pts with BL structural damage presence (ES > 0) vs absence (ES = 0), or high (ES ≥ 3) vs low (ES < 3) structural damage, in either arm and between ABA + MTX vs PBO + MTX arms. Outcome measures at wk 52 included: proportions of pts with ES increase ≥ 1 and by subgroups (increases of 1, 2, ≥ 3); change from BL in ES; and clinical outcomes (Simplified Disease Activity Index [SDAI] remission [≤ 3.3], DAS28 [CRP] < 2.6, and clinical improvement defined as ≥ 80% improvement in SDAI or DAS28 [CRP]).
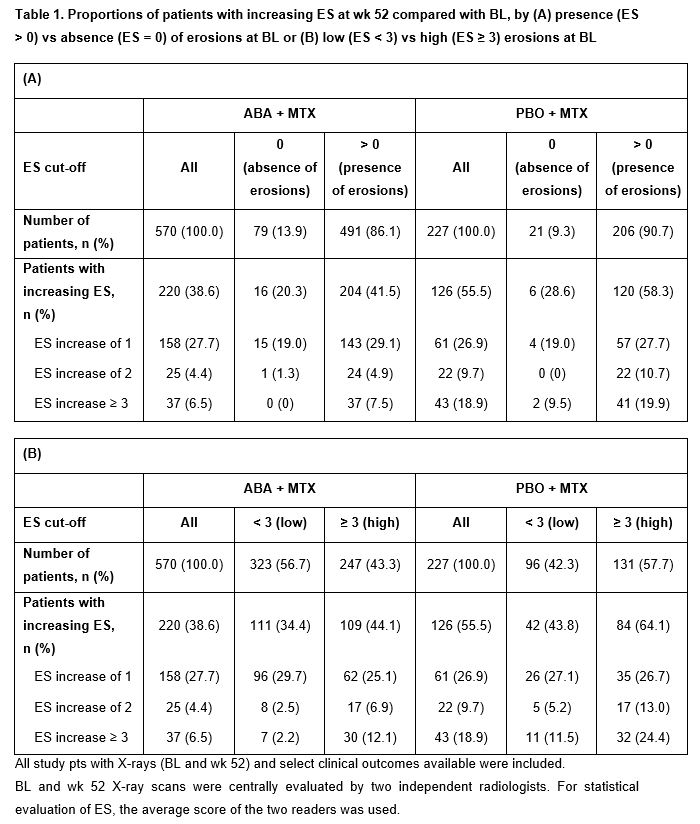
Results: Pts with BL erosions were more likely to have new erosions at wk 52 vs pts without BL erosions in both the ABA + MTX (41.5 vs 20.3%) and PBO + MTX (58.3 vs 28.6%) arms; rates were numerically lower with ABA + MTX vs PBO + MTX (Table 1A). Rates of new erosions were also higher in those with ES ≥ 3 (vs < 3) at BL, and were numerically lower in each subgroup with ABA vs PBO (Table 1B). In the BL erosion present (Table 1A) or high BL erosion groups (Table 1B), the proportions of pts with ES increasing by ≥ 3 in the PBO + MTX arm were at least twice as high as in the ABA + MTX arm. Overall, progression of structural damage was less pronounced in pts in the ABA + MTX vs PBO + MTX arm (ES increase 1–2: 4.4 vs 9.7%, respectively; ES increase ≥ 3: 6.5 vs 18.9%, respectively; Table 1A, 1B).
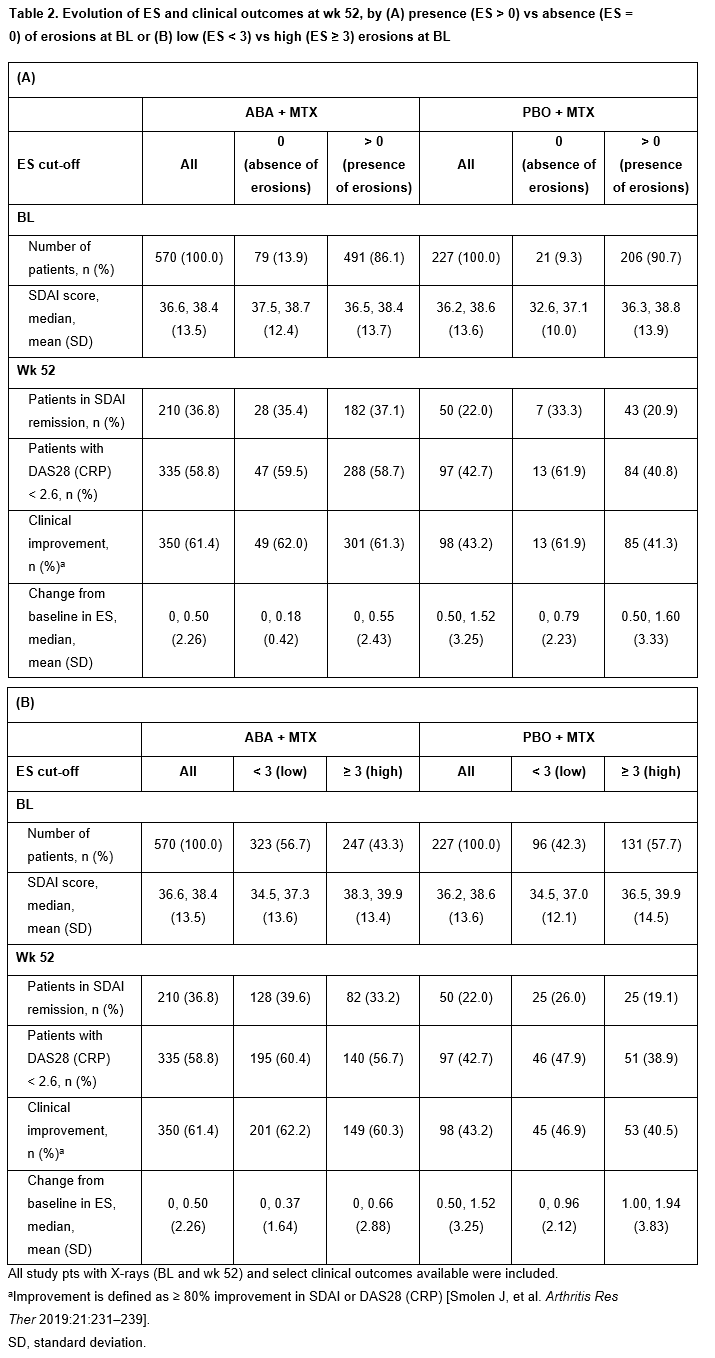
Changes in ES from BL to wk 52 and clinical outcomes at wk 52 are shown for pts by BL ES presence/absence (Table 2A) and high/low status (Table 2B). At wk 52, SDAI remission rates were higher in low vs high BL ES groups in both arms (Table 2B). Overall, clinical response rates were higher in the ABA + MTX vs PBO + MTX arm (SDAI remission: 36.8 vs 22.0%, respectively; DAS28 (CRP) < 2.6: 58.8 vs 42.7%, respectively; clinical improvement: 61.4 vs 43.2%, respectively; Table 2A, 2B).
Conclusion: In ACPA+ pts with early RA, abatacept + MTX was more effective than MTX alone in slowing the progression of structural damage and improving clinical response over 52 wks, with greater progression of structural damage associated with presence and severity of BL erosions.
The greater progression of structural damage in pts with high BL erosions receiving MTX alone vs abatacept + MTX reinforces the need to treat such pts earlier with biologic DMARDs such as abatacept to better preserve their joints.
1Emery P, et al. Arthritis Rheum 2018;70.abs563.
2Aletaha D, et al. Arthritis Rheum 2010;62:2569–2581.
Medical writing: Joanna Wright (Caudex), funded by Bristol Myers Squibb.
Disclosures: C. Pachai, Bristol Myers Squibb; S. Connolly, Bristol Myers Squibb; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; V. Bykerk, Janssen, Bristol Myers Squibb, Crossbridge, Pfizer, Sanofi Aventis, Brainstorm Therapeutics, Amgen, UCB, Gilead, Genzyme Corporation, Regeneron; T. Huizinga, None; G. Citera, AbbVie, Amgen, Bristol Myers Squibb, Gema, Janssen, Pfizer, Sandoz, Boehringer Ingelheim; C. Bingham III, AbbVie, Janssen, Pfizer, Sanofi, Bristol Myers Squibb, Eli Lilly; S. Du, Bristol Myers Squibb; G. Hoexter, Bristol Myers Squibb GmbH & Co. KGaA; R. Fleischmann, AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galvani, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, UCB, Galapagos; S. Banerjee, Bristol Myers Squibb; P. Emery, AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Novartis, Pfizer, Roche, Samsung.
Background/Purpose: Presence of structural damage is an important risk factor for further damage progression over time in RA. Inhibition of damage progression is a key outcome measure in RA trials.
We assessed the effect of baseline (BL) structural damage, based on X-ray erosion scores (ES), on the progression of structural damage and clinical response over 52 weeks (wks) with subcutaneous (SC) abatacept (ABA) treatment in MTX-naïve, ACPA+ patients (pts) with early RA in the phase 3b AVERT-2 study (NCT02504268).1
Methods: Pts with early RA (ACR/EULAR 2010)2 were randomized to weekly SC ABA 125 mg or SC placebo (PBO), both with oral MTX, for 56 wks. X-rays were evaluated using modified total Sharp/van der Heijde score. This retrospective analysis included all pts with X-rays (BL and wk 52) and select clinical outcomes available.
Comparisons were made between pts with BL structural damage presence (ES > 0) vs absence (ES = 0), or high (ES ≥ 3) vs low (ES < 3) structural damage, in either arm and between ABA + MTX vs PBO + MTX arms. Outcome measures at wk 52 included: proportions of pts with ES increase ≥ 1 and by subgroups (increases of 1, 2, ≥ 3); change from BL in ES; and clinical outcomes (Simplified Disease Activity Index [SDAI] remission [≤ 3.3], DAS28 [CRP] < 2.6, and clinical improvement defined as ≥ 80% improvement in SDAI or DAS28 [CRP]).
Results: Pts with BL erosions were more likely to have new erosions at wk 52 vs pts without BL erosions in both the ABA + MTX (41.5 vs 20.3%) and PBO + MTX (58.3 vs 28.6%) arms; rates were numerically lower with ABA + MTX vs PBO + MTX (Table 1A). Rates of new erosions were also higher in those with ES ≥ 3 (vs < 3) at BL, and were numerically lower in each subgroup with ABA vs PBO (Table 1B). In the BL erosion present (Table 1A) or high BL erosion groups (Table 1B), the proportions of pts with ES increasing by ≥ 3 in the PBO + MTX arm were at least twice as high as in the ABA + MTX arm. Overall, progression of structural damage was less pronounced in pts in the ABA + MTX vs PBO + MTX arm (ES increase 1–2: 4.4 vs 9.7%, respectively; ES increase ≥ 3: 6.5 vs 18.9%, respectively; Table 1A, 1B).
Changes in ES from BL to wk 52 and clinical outcomes at wk 52 are shown for pts by BL ES presence/absence (Table 2A) and high/low status (Table 2B). At wk 52, SDAI remission rates were higher in low vs high BL ES groups in both arms (Table 2B). Overall, clinical response rates were higher in the ABA + MTX vs PBO + MTX arm (SDAI remission: 36.8 vs 22.0%, respectively; DAS28 (CRP) < 2.6: 58.8 vs 42.7%, respectively; clinical improvement: 61.4 vs 43.2%, respectively; Table 2A, 2B).
Conclusion: In ACPA+ pts with early RA, abatacept + MTX was more effective than MTX alone in slowing the progression of structural damage and improving clinical response over 52 wks, with greater progression of structural damage associated with presence and severity of BL erosions.
The greater progression of structural damage in pts with high BL erosions receiving MTX alone vs abatacept + MTX reinforces the need to treat such pts earlier with biologic DMARDs such as abatacept to better preserve their joints.
1Emery P, et al. Arthritis Rheum 2018;70.abs563.
2Aletaha D, et al. Arthritis Rheum 2010;62:2569–2581.
Medical writing: Joanna Wright (Caudex), funded by Bristol Myers Squibb.


Disclosures: C. Pachai, Bristol Myers Squibb; S. Connolly, Bristol Myers Squibb; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; V. Bykerk, Janssen, Bristol Myers Squibb, Crossbridge, Pfizer, Sanofi Aventis, Brainstorm Therapeutics, Amgen, UCB, Gilead, Genzyme Corporation, Regeneron; T. Huizinga, None; G. Citera, AbbVie, Amgen, Bristol Myers Squibb, Gema, Janssen, Pfizer, Sandoz, Boehringer Ingelheim; C. Bingham III, AbbVie, Janssen, Pfizer, Sanofi, Bristol Myers Squibb, Eli Lilly; S. Du, Bristol Myers Squibb; G. Hoexter, Bristol Myers Squibb GmbH & Co. KGaA; R. Fleischmann, AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galvani, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, UCB, Galapagos; S. Banerjee, Bristol Myers Squibb; P. Emery, AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Novartis, Pfizer, Roche, Samsung.

