Back
Ignite Talk
Session: Ignite Session 6B
1998: Effect of a Lifestyle Program Based on a Whole Food Plant-based Diet, Physical Activity, and Stress Management in Patients with Rheumatoid Arthritis: A Randomized Controlled Trial
Sunday, November 13, 2022
2:20 PM – 2:25 PM Eastern Time
Location: Center City Stage
- WW
Wendy Walrabenstein, MA
Amsterdam UMC
Amsterdam, NetherlandsDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Wendy Walrabenstein1, Carlijn Wagenaar1, Marike van der Leeden2, Franktien Turkstra1, Jos Twisk3, Maarten Boers4, Henriët van Middendorp5, Peter Weijs2 and Dirkjan van Schaardenburg2, 1Reade Rheumatology Center, Amsterdam, Netherlands, 2Amsterdam UMC, Amsterdam, Netherlands, 3Department of Epidemiology and Biostatistics, Amsterdam, Netherlands, 4Amsterdam UMC, Vrije Universiteit, Amsterdam, Netherlands, 5Leiden University, Leiden, Netherlands
Background/Purpose: Lifestyle factors have been associated with the development and progression of rheumatoid arthritis. Interventions involving whole food plant-based diets (1-3), physical activity (4) or stress management (5) have shown promising results for people with rheumatoid arthritis but were not yet evaluated in an integrated program. We therefore aimed to determine the effect of a 16-week multidisciplinary lifestyle program on disease activity in patients with rheumatoid arthritis with low to moderate disease activity.
Methods: In the "Plants for Joints" parallel-arm, assessor-blind randomized clinical trial, patients with rheumatoid arthritis and a 28-joint Disease Activity Score [DAS28] score ≥ 2.6 and ≤ 5.1, were assigned to the "Plants for Joints" group or the control group. The "Plants for Joints" group followed a lifestyle program based on a whole food plant-based diet, physical activity, and stress management in addition to usual care. The control group received usual care. Medication was kept stable three months before and during the trial. Secondary outcomes included anthropometric, and metabolic markers. An intention-to-treat analysis with a linear mixed model, adjusted for baseline values was used to analyze between-group differences of continuous outcomes.
Results: Of 115 people screened, 83 were randomized and 77 completed the study. Participants were 92% female with a mean (SD) age of 55 (12) and body mass index of 26 (4) kg/m2. After 16 weeks the "Plants for Joints" group had a mean 0.9-point greater improvement of the DAS28 versus the control group (95% CI 0.4 to 1.3; p < 0.0001) (Figure 1). Subgroup analyses showed significant improvements in the seropositive as well as the seronegative subgroup, although the effect was more profound in the seronegative group. Weight, fat mass, HbA1c, LDL and triglycerides also showed significant improvements in the "Plants for Joints" versus control group, while blood glucose and HDL remained unchanged (Table 1). No serious adverse events occurred.
Conclusion: The 16-week "Plants for Joints" lifestyle program substantially decreased disease activity in people with rheumatoid arthritis with low to moderate disease activity.
References:
1. Kjeldsen-Kragh et al. Lancet, 1991.
2. Hafstrom et al. Rheumatology (Oxford), 2001.
3. Skoldstam et al. Ann Rheum Dis, 2003.
4. Hurkmans et al. Cochrane Database Syst Rev, 2009.
5. De Brouwer et al. Arthritis Res Ther, 2013.
.jpg) All values for the total group (n = 77), except the DAS28 for the seropositive and seronegative group. Seropositive subjects are positive for rheumatoid factor [RF] or anti-citrullinated protein antibodies [ACPA], seronegative subjects are negative for RF and ACPA. DAS28 = 28-joint disease activity score, ESR = erythrocyte sedimentation rate, patient's self reported global assessment of disease activity is based on a visual analogue scale (0-100 mm) with higher values indicating more disease activity, EULAR Good Response = improvement DAS28 > 1.2 and DAS28 ≤ 3.2 (non-responders include responders EULAR Moderate Response), CRP = C-reactive protein, DEXA = Dual-energy X-ray absorptiometry, SD = standard deviation, CI = confidence interval, OR = odds ratio.
All values for the total group (n = 77), except the DAS28 for the seropositive and seronegative group. Seropositive subjects are positive for rheumatoid factor [RF] or anti-citrullinated protein antibodies [ACPA], seronegative subjects are negative for RF and ACPA. DAS28 = 28-joint disease activity score, ESR = erythrocyte sedimentation rate, patient's self reported global assessment of disease activity is based on a visual analogue scale (0-100 mm) with higher values indicating more disease activity, EULAR Good Response = improvement DAS28 > 1.2 and DAS28 ≤ 3.2 (non-responders include responders EULAR Moderate Response), CRP = C-reactive protein, DEXA = Dual-energy X-ray absorptiometry, SD = standard deviation, CI = confidence interval, OR = odds ratio.
(a) Odds ratio calculation based on logistic generalized estimating equation (GEE) regression analysis of change between baseline and 16 weeks.
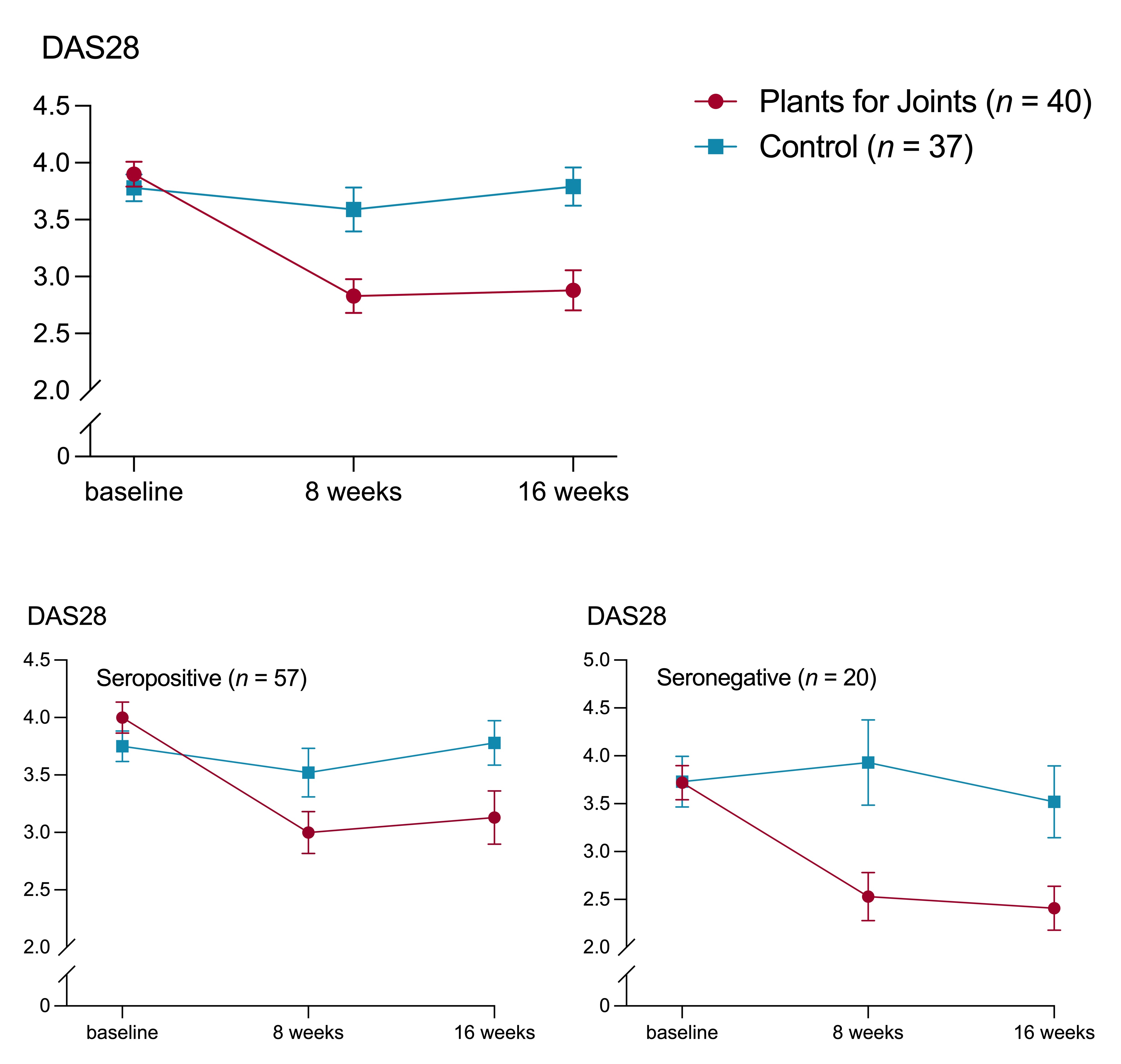 Change in disease activity score based on 28 joints (DAS28, primary outcome) for the total group (p < 0.0001) is presented in the top panel with mean ± standard error. The panels below present the same for seropositive subjects (positive for rheumatoid factor [RF] and/or anti-citrullinated protein antibodies [ACPA], p < 0.01) and seronegative subjects (negative for RF and ACPA, p < 0.01).
Change in disease activity score based on 28 joints (DAS28, primary outcome) for the total group (p < 0.0001) is presented in the top panel with mean ± standard error. The panels below present the same for seropositive subjects (positive for rheumatoid factor [RF] and/or anti-citrullinated protein antibodies [ACPA], p < 0.01) and seronegative subjects (negative for RF and ACPA, p < 0.01).
Disclosures: W. Walrabenstein, None; C. Wagenaar, None; M. van der Leeden, None; F. Turkstra, None; J. Twisk, None; M. Boers, Novartis; H. van Middendorp, None; P. Weijs, None; D. van Schaardenburg, None.
Background/Purpose: Lifestyle factors have been associated with the development and progression of rheumatoid arthritis. Interventions involving whole food plant-based diets (1-3), physical activity (4) or stress management (5) have shown promising results for people with rheumatoid arthritis but were not yet evaluated in an integrated program. We therefore aimed to determine the effect of a 16-week multidisciplinary lifestyle program on disease activity in patients with rheumatoid arthritis with low to moderate disease activity.
Methods: In the "Plants for Joints" parallel-arm, assessor-blind randomized clinical trial, patients with rheumatoid arthritis and a 28-joint Disease Activity Score [DAS28] score ≥ 2.6 and ≤ 5.1, were assigned to the "Plants for Joints" group or the control group. The "Plants for Joints" group followed a lifestyle program based on a whole food plant-based diet, physical activity, and stress management in addition to usual care. The control group received usual care. Medication was kept stable three months before and during the trial. Secondary outcomes included anthropometric, and metabolic markers. An intention-to-treat analysis with a linear mixed model, adjusted for baseline values was used to analyze between-group differences of continuous outcomes.
Results: Of 115 people screened, 83 were randomized and 77 completed the study. Participants were 92% female with a mean (SD) age of 55 (12) and body mass index of 26 (4) kg/m2. After 16 weeks the "Plants for Joints" group had a mean 0.9-point greater improvement of the DAS28 versus the control group (95% CI 0.4 to 1.3; p < 0.0001) (Figure 1). Subgroup analyses showed significant improvements in the seropositive as well as the seronegative subgroup, although the effect was more profound in the seronegative group. Weight, fat mass, HbA1c, LDL and triglycerides also showed significant improvements in the "Plants for Joints" versus control group, while blood glucose and HDL remained unchanged (Table 1). No serious adverse events occurred.
Conclusion: The 16-week "Plants for Joints" lifestyle program substantially decreased disease activity in people with rheumatoid arthritis with low to moderate disease activity.
References:
1. Kjeldsen-Kragh et al. Lancet, 1991.
2. Hafstrom et al. Rheumatology (Oxford), 2001.
3. Skoldstam et al. Ann Rheum Dis, 2003.
4. Hurkmans et al. Cochrane Database Syst Rev, 2009.
5. De Brouwer et al. Arthritis Res Ther, 2013.
.jpg) All values for the total group (n = 77), except the DAS28 for the seropositive and seronegative group. Seropositive subjects are positive for rheumatoid factor [RF] or anti-citrullinated protein antibodies [ACPA], seronegative subjects are negative for RF and ACPA. DAS28 = 28-joint disease activity score, ESR = erythrocyte sedimentation rate, patient's self reported global assessment of disease activity is based on a visual analogue scale (0-100 mm) with higher values indicating more disease activity, EULAR Good Response = improvement DAS28 > 1.2 and DAS28 ≤ 3.2 (non-responders include responders EULAR Moderate Response), CRP = C-reactive protein, DEXA = Dual-energy X-ray absorptiometry, SD = standard deviation, CI = confidence interval, OR = odds ratio.
All values for the total group (n = 77), except the DAS28 for the seropositive and seronegative group. Seropositive subjects are positive for rheumatoid factor [RF] or anti-citrullinated protein antibodies [ACPA], seronegative subjects are negative for RF and ACPA. DAS28 = 28-joint disease activity score, ESR = erythrocyte sedimentation rate, patient's self reported global assessment of disease activity is based on a visual analogue scale (0-100 mm) with higher values indicating more disease activity, EULAR Good Response = improvement DAS28 > 1.2 and DAS28 ≤ 3.2 (non-responders include responders EULAR Moderate Response), CRP = C-reactive protein, DEXA = Dual-energy X-ray absorptiometry, SD = standard deviation, CI = confidence interval, OR = odds ratio.(a) Odds ratio calculation based on logistic generalized estimating equation (GEE) regression analysis of change between baseline and 16 weeks.
 Change in disease activity score based on 28 joints (DAS28, primary outcome) for the total group (p < 0.0001) is presented in the top panel with mean ± standard error. The panels below present the same for seropositive subjects (positive for rheumatoid factor [RF] and/or anti-citrullinated protein antibodies [ACPA], p < 0.01) and seronegative subjects (negative for RF and ACPA, p < 0.01).
Change in disease activity score based on 28 joints (DAS28, primary outcome) for the total group (p < 0.0001) is presented in the top panel with mean ± standard error. The panels below present the same for seropositive subjects (positive for rheumatoid factor [RF] and/or anti-citrullinated protein antibodies [ACPA], p < 0.01) and seronegative subjects (negative for RF and ACPA, p < 0.01).Disclosures: W. Walrabenstein, None; C. Wagenaar, None; M. van der Leeden, None; F. Turkstra, None; J. Twisk, None; M. Boers, Novartis; H. van Middendorp, None; P. Weijs, None; D. van Schaardenburg, None.

