Back
Ignite Talk
Session: Ignite Session 6A
0880: Impact of Characteristic Inflammatory and Structural Imaging Lesions on Expert Classification of Axial Juvenile Spondyloarthritis
Sunday, November 13, 2022
2:45 PM – 2:50 PM Eastern Time
Location: Northern Liberties Stage
- AM
Adam Mayer, MD
University of Pennsylvania/Children's Hospital of Philadelphia
Philadelphia, PA, United StatesDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Adam Mayer1, Timothy Brandon1, Amita Aggarwal2, Ruben Burgos vargas3, Robert Colbert4, Gerd Horneff5, Rik Joos6, Ronald Laxer7, Kirsten Minden8, Angelo Ravelli9, Nicola Ruperto10, Judith Smith11, Matthew Stoll12, Shirley Tse7, Filip Van den bosch13, Walter P Maksymowych14, Robert G Lambert15, David Biko16, Nancy Chauvin17, Michael Francavilla16, Jacob Jaremko15, Nele Herregods18, Jennifer Faerber1, Ozgur Kasapcopur19, Mehmet YILDIZ20, Alison Hendry21 and Pamela Weiss22, 1Children's Hospital of Philadelphia, Philadelphia, PA, 2Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, 3hospital general de mexico, Ciudad de México, Mexico, 4NIH/NIAMS, Bethesda, MD, 5Pediatrics, Asklepios Klinik Sankt Augustin GmbH, Sankt Augustin, Germany, 6Ghent University Hospital, Basel, Switzerland, 7Division of Rheumatology, The Hospital for Sick Children; Child Health Evaluative Services, SickKids Research Institute; Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 8Charité Universitätsmedizin Berlin, Berlin, Germany, 9Department of Neurosciences, Rehabilitation, Ophthalmology, Genetic and Maternal Infantile Sciences (DINOGMI) University of Genoa, Italy,Scientific Direction, IRCCS Istituto Giannina Gaslini, Genova, Italy, 10IRCCS Istituto Giannina Gaslini; PRINTO, Clinica Pediatrica e Reumatologia, Genova, Italy, 11University of Wisconsin, Madison, WI, 12University of Alabama at Birmingham, Birmingham, AL, 13Department of Internal Medicine and Paediatrics, Ghent University and VIB Centre for Inflammation Research, Ghent, Belgium, 14Department of Medicine, University of Alberta, Edmonton, AB, Canada, 15University of Alberta, Edmonton, AB, Canada, 16University of Pennsylvania, Philadelphia, PA, 17Penn State Health, Hershey, PA, 18Ghent University Hospital, Ghent, Belgium, 19Istanbul University-Cerrahpaşa, Cerrahpaşa Medical School, Istanbul, Turkey, 20Istanbul University-Cerrahpaşa, Istanbul, Turkey, 21Middlemore Hospital, Auckland, 22Children's Hospital of Philadelphia, Glen Mills, PA
Background/Purpose: Axial disease in juvenile spondyloarthritis (JSpA) is difficult to assess in children and the role of MRI in rheumatologist diagnosis has not yet been quantified. We aimed to quantify the impact of MRI findings on high confidence in classification of axial disease and to assess the independent association of clinical factors and MRI findings with expert classification of axial JSpA.
Methods: Clinical characteristics for JSpA cases with suspected axial disease from 6 international centers were collected on a case report form; all cases had an MRI performed. Imaging was independently rated for the presence/absence of inflammatory or structural lesions typical of axial disease by at least two raters from a team of imaging experts. SpA clinical experts (n=14) completed two assessments for each case: one based only on clinical features and the other with inclusion of MRI findings. Each case was reviewed by 3 SpA clinical experts who rated their confidence for presence of "axial JSpA" from -3 (very unlikely) to +3 (very likely). High confidence was defined as ≤-2 or ≥+2 and agreement was defined as ≥2 raters with high confidence. Univariable and multivariable logistic regressions were conducted to assess associations with high confidence in classification of axial disease through odds ratios (OR) and confidence intervals (CI) for both assessments. The multivariable logistic regression model for both assessments was built using the best subsets algorithm to identify the best subsets of clinical and/or imaging characteristics to include in the model. Area under the curve (AUC) and clinical acumen were used to select the highest performing model for each assessment.
Results: Among the 303 cases, agreement was achieved on 131 (43%) cases with clinical factors and on 214 (71%) cases with clinical factors plus central imaging assessment (Figure 1). Adding central imaging data affected high confidence agreement in the presence/absence of axial disease for 47% (144/304) of cases; addition of central MR imaging data facilitated agreement on 113 cases but lost agreement on 31. Of the 113 cases gaining agreement, 43 and 70 reached high confidence agreement that the case was or was not axial disease, respectively. For 18 (5.9%) cases, it changed the directionality of agreement; 5 cases from axial disease present to axial disease absent and 13 cases from not axial disease to axial disease present. Table 1 shows the results of the regression analyses for both expert assessments. The multivariable logistic regression model using clinical factors alone as predictors found that HLA-B27+, lumbar spinal pain and pain that improved with activity had the strongest associations with axial disease classification. The analysis including imaging data found that presence of SIJ structural or inflammatory lesions on MRI had strong and independent associations with classification of axial disease by SpA experts; clinical factors other than pain improvement with activity added little to no incremental value in model fit.
Conclusion: The addition of MR imaging facilitates expert agreement on classification of axial disease in JSpA and in multivariate analysis minimizes the incremental value and strength of association of several clinical domains.
.jpg)
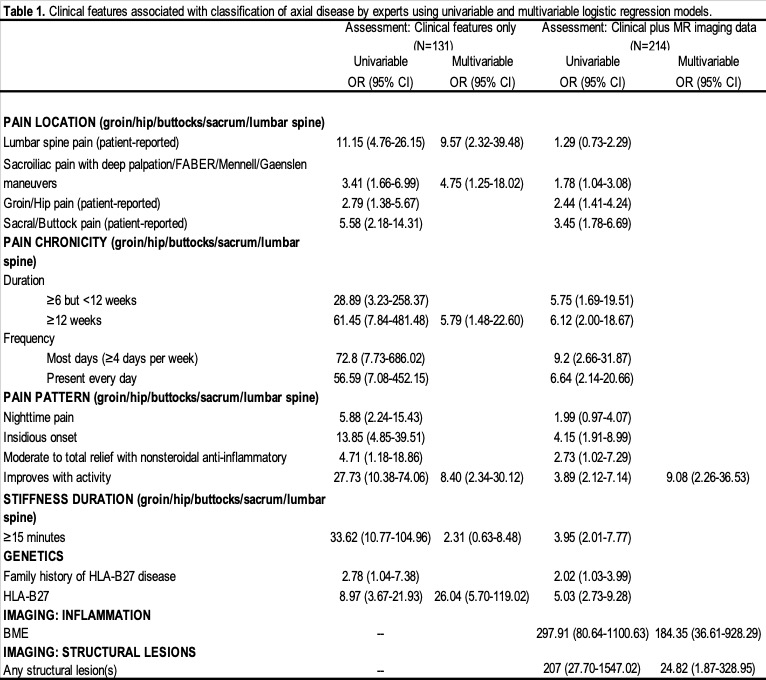 Univariable and multivariable regressions were conducted to assess associations with high confidence in classification of axial disease through odds ratios (OR) and confidence intervals (CI) for assessment of clinical features only and assessment of clinical plus imaging features.
Univariable and multivariable regressions were conducted to assess associations with high confidence in classification of axial disease through odds ratios (OR) and confidence intervals (CI) for assessment of clinical features only and assessment of clinical plus imaging features.
Disclosures: A. Mayer, None; T. Brandon, None; A. Aggarwal, None; R. Burgos vargas, None; R. Colbert, Eli Lilly; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott; R. Joos, None; R. Laxer, Eli Lilly, Novartis, sanofi, sobi; K. Minden, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Roche, Biogen; A. Ravelli, None; N. Ruperto, 2 Bridge, Amgen, AstraZeneca, Aurinia, Bayer, Brystol Myers and Squibb, Celgene, inMed, Cambridge Healthcare Research, Domain Therapeutic,, EMD Serono, Glaxo Smith Kline, Idorsia, Janssen, Eli Lilly, Novartis, Pfizer, Sobi, UCB; J. Smith, None; M. Stoll, Novartis; S. Tse, None; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; R. Lambert, Calyx, CARE Arthritis, Image Analysis Group; D. Biko, None; N. Chauvin, None; M. Francavilla, None; J. Jaremko, None; N. Herregods, None; J. Faerber, None; O. Kasapcopur, None; M. YILDIZ, None; A. Hendry, None; P. Weiss, None.
Background/Purpose: Axial disease in juvenile spondyloarthritis (JSpA) is difficult to assess in children and the role of MRI in rheumatologist diagnosis has not yet been quantified. We aimed to quantify the impact of MRI findings on high confidence in classification of axial disease and to assess the independent association of clinical factors and MRI findings with expert classification of axial JSpA.
Methods: Clinical characteristics for JSpA cases with suspected axial disease from 6 international centers were collected on a case report form; all cases had an MRI performed. Imaging was independently rated for the presence/absence of inflammatory or structural lesions typical of axial disease by at least two raters from a team of imaging experts. SpA clinical experts (n=14) completed two assessments for each case: one based only on clinical features and the other with inclusion of MRI findings. Each case was reviewed by 3 SpA clinical experts who rated their confidence for presence of "axial JSpA" from -3 (very unlikely) to +3 (very likely). High confidence was defined as ≤-2 or ≥+2 and agreement was defined as ≥2 raters with high confidence. Univariable and multivariable logistic regressions were conducted to assess associations with high confidence in classification of axial disease through odds ratios (OR) and confidence intervals (CI) for both assessments. The multivariable logistic regression model for both assessments was built using the best subsets algorithm to identify the best subsets of clinical and/or imaging characteristics to include in the model. Area under the curve (AUC) and clinical acumen were used to select the highest performing model for each assessment.
Results: Among the 303 cases, agreement was achieved on 131 (43%) cases with clinical factors and on 214 (71%) cases with clinical factors plus central imaging assessment (Figure 1). Adding central imaging data affected high confidence agreement in the presence/absence of axial disease for 47% (144/304) of cases; addition of central MR imaging data facilitated agreement on 113 cases but lost agreement on 31. Of the 113 cases gaining agreement, 43 and 70 reached high confidence agreement that the case was or was not axial disease, respectively. For 18 (5.9%) cases, it changed the directionality of agreement; 5 cases from axial disease present to axial disease absent and 13 cases from not axial disease to axial disease present. Table 1 shows the results of the regression analyses for both expert assessments. The multivariable logistic regression model using clinical factors alone as predictors found that HLA-B27+, lumbar spinal pain and pain that improved with activity had the strongest associations with axial disease classification. The analysis including imaging data found that presence of SIJ structural or inflammatory lesions on MRI had strong and independent associations with classification of axial disease by SpA experts; clinical factors other than pain improvement with activity added little to no incremental value in model fit.
Conclusion: The addition of MR imaging facilitates expert agreement on classification of axial disease in JSpA and in multivariate analysis minimizes the incremental value and strength of association of several clinical domains.
.jpg)
 Univariable and multivariable regressions were conducted to assess associations with high confidence in classification of axial disease through odds ratios (OR) and confidence intervals (CI) for assessment of clinical features only and assessment of clinical plus imaging features.
Univariable and multivariable regressions were conducted to assess associations with high confidence in classification of axial disease through odds ratios (OR) and confidence intervals (CI) for assessment of clinical features only and assessment of clinical plus imaging features.Disclosures: A. Mayer, None; T. Brandon, None; A. Aggarwal, None; R. Burgos vargas, None; R. Colbert, Eli Lilly; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott; R. Joos, None; R. Laxer, Eli Lilly, Novartis, sanofi, sobi; K. Minden, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Roche, Biogen; A. Ravelli, None; N. Ruperto, 2 Bridge, Amgen, AstraZeneca, Aurinia, Bayer, Brystol Myers and Squibb, Celgene, inMed, Cambridge Healthcare Research, Domain Therapeutic,, EMD Serono, Glaxo Smith Kline, Idorsia, Janssen, Eli Lilly, Novartis, Pfizer, Sobi, UCB; J. Smith, None; M. Stoll, Novartis; S. Tse, None; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; R. Lambert, Calyx, CARE Arthritis, Image Analysis Group; D. Biko, None; N. Chauvin, None; M. Francavilla, None; J. Jaremko, None; N. Herregods, None; J. Faerber, None; O. Kasapcopur, None; M. YILDIZ, None; A. Hendry, None; P. Weiss, None.

