Back
Poster Session D
Session: (2154–2178) Systemic Sclerosis and Related Disorders – Clinical Poster III
2158: A Decade of Caring for SSc Patients: Do Outcomes Improve? Data from the Leiden CCISS Cohort
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SL
Sophie Liem, MD
LUMC
Leiden, Netherlands
Abstract Poster Presenter(s)
Sophie Liem1, Saad Ahmed2, Jacopo Ciaffi3, Liesbeth Beaart-van de Voorde4, Anne Schouffoer5, J Geelhoed6, Nina Ajmone Marsan7, Tom Huizinga8 and Jeska de Vries-Bouwstra8, 1LUMC, Leiden, Netherlands, 2Leiden University Medical Centre, Leiden, Netherlands, 3IRCCS Istituto Ortopedico Rizzoli, Bologna/Leiden University Medical Center, Bologna, Italy, 4Leiden University Medical Center, Leiden, Netherlands, 5Leiden University Medical Center/Haga Teaching Hospital, Leiden, Netherlands, 6Leiden University Medical Center, Pulmonology, Leiden, Zuid-Holland, Netherlands, 7Leiden University Medical Center, Cardiology, Leiden, Netherlands, 8Leiden University Medical Center, Leiden, Netherlands
Background/Purpose: Combined Care in Systemic Sclerosis (CCISS) is a prospective cohort of patients referred to Leiden University Medical Center for Raynaud's Phenomenon (RP), a suspicion of systemic sclerosis (SSc) or a connective tissue disease. This cohort is characterized by its standardized and extensive annual follow-up. Since its beginning in 2009, diagnostic criteria for SSc have been updated leading to a higher sensitivity for early SSc (ACR 2013 criteria). This study aimed to determine whether disease characteristics and mortality of SSc patients changed over time, with cohort entry year as instrumental variable, for all patients, male and female patients, and anti-topoisomerase (ATA) and anticentromere antibodies (ACA), separately.
Methods: 643 SSc-patients fulfilling ACR/EULAR 2013 SSc criteria, were included, and categorized into three groups based on cohort entry year: 1) 2010–2013 (n=229,36%), 2) 2014–2017 (n=207,32%), and 3) 2018–2021 (n=207,32%). Characteristics included disease duration (=months since RP, first non-RP symptom and first date of diagnosis by a physician), interstitial lung disease (ILD; =presence of ILD on HRCT and FVC< 80%), pulmonary arterial hypertension, digital ulcers (DU), diffuse cutaneous SSc (dcSSc), ATA and ACA. Characteristics were compared between cohort entry groups, using appropriate tests. Survival for a 3-year follow-up period was evaluated and compared between the groups.
Results: 643 SSc patients were included in this study, of whom n=229 (36%) from 2010–2013, 207 (32%) from 2014–2017, and 207 (32%) from 2018–2021. Median follow-up duration was 4 years.
When comparing disease characteristics between the cohort entry groups we observed several important trends over time. First, the proportion of male patients increased. Second, disease duration before cohort entry decreased, reflected by a decrease in RP and non-RP duration. Of the investigated organ complications we observed a decrease in patients presenting with DU, while a trend for decrease in patients presenting with ILD and dcSSc was observed (Table 1). Overall 3-year survival was 94% and did not differ between the cohort entry groups (Figure 1).
When stratifying for sex, we observed that disease duration at cohort entry decreased over time in both males and females, but was always longer in females than in males. Survival was worse in males than in females for all groups (89 vs 95%, Figure 1).
When stratifying for autoantibody, presence of DU at cohort entry decreased significantly over time in ACA+ patients, whereas for ATA+ patients this remained stable. Notably, almost no ACA+ patients had ILD at cohort entry in all groups, while ATA+ patients had a proportion of 25% in 2010-2013 which decreased to 19% in 2018-2021.
Conclusion: Over 10 years of follow-up we observe a decrease in duration between SSc diagnosis and cohort entry, which might indicate more timely diagnosis of SSc. Although trends for less severe organ complications at baseline are observed, no change in 3-year survival is found. Specifically, survival in males is importantly worse than in females, underlining the need for better understanding of disease progression and more effective treatment.
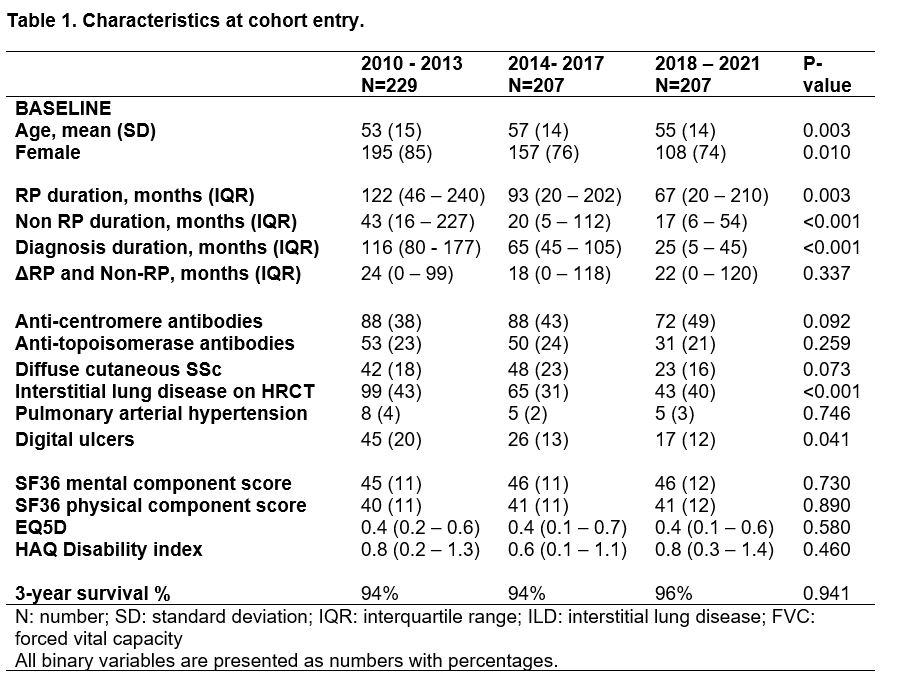 Table 1. Cohort entry characteristics of included SSc patients from the Leiden CCISS cohort
Table 1. Cohort entry characteristics of included SSc patients from the Leiden CCISS cohort
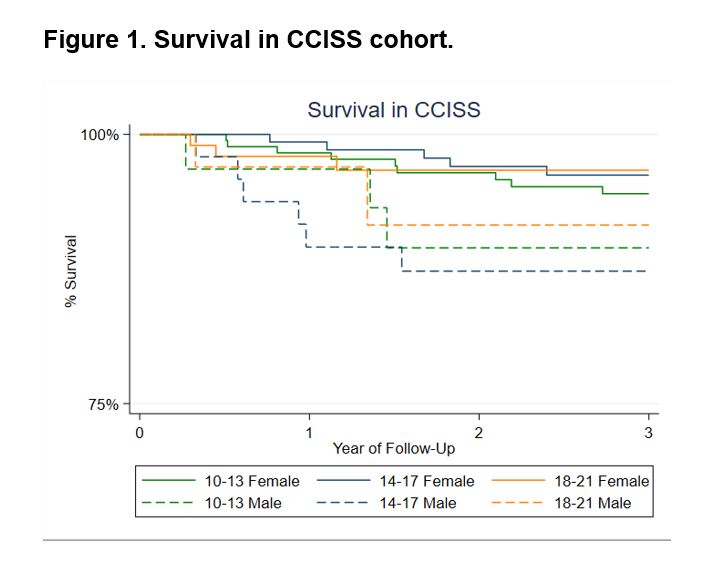 Figure 1. Survival in CCISS cohort.
Figure 1. Survival in CCISS cohort.
Disclosures: S. Liem, None; S. Ahmed, Janssen pharamaceuticals; J. Ciaffi, None; L. Beaart-van de Voorde, None; A. Schouffoer, None; J. Geelhoed, None; N. Ajmone Marsan, None; T. Huizinga, None; J. de Vries-Bouwstra, None.
Background/Purpose: Combined Care in Systemic Sclerosis (CCISS) is a prospective cohort of patients referred to Leiden University Medical Center for Raynaud's Phenomenon (RP), a suspicion of systemic sclerosis (SSc) or a connective tissue disease. This cohort is characterized by its standardized and extensive annual follow-up. Since its beginning in 2009, diagnostic criteria for SSc have been updated leading to a higher sensitivity for early SSc (ACR 2013 criteria). This study aimed to determine whether disease characteristics and mortality of SSc patients changed over time, with cohort entry year as instrumental variable, for all patients, male and female patients, and anti-topoisomerase (ATA) and anticentromere antibodies (ACA), separately.
Methods: 643 SSc-patients fulfilling ACR/EULAR 2013 SSc criteria, were included, and categorized into three groups based on cohort entry year: 1) 2010–2013 (n=229,36%), 2) 2014–2017 (n=207,32%), and 3) 2018–2021 (n=207,32%). Characteristics included disease duration (=months since RP, first non-RP symptom and first date of diagnosis by a physician), interstitial lung disease (ILD; =presence of ILD on HRCT and FVC< 80%), pulmonary arterial hypertension, digital ulcers (DU), diffuse cutaneous SSc (dcSSc), ATA and ACA. Characteristics were compared between cohort entry groups, using appropriate tests. Survival for a 3-year follow-up period was evaluated and compared between the groups.
Results: 643 SSc patients were included in this study, of whom n=229 (36%) from 2010–2013, 207 (32%) from 2014–2017, and 207 (32%) from 2018–2021. Median follow-up duration was 4 years.
When comparing disease characteristics between the cohort entry groups we observed several important trends over time. First, the proportion of male patients increased. Second, disease duration before cohort entry decreased, reflected by a decrease in RP and non-RP duration. Of the investigated organ complications we observed a decrease in patients presenting with DU, while a trend for decrease in patients presenting with ILD and dcSSc was observed (Table 1). Overall 3-year survival was 94% and did not differ between the cohort entry groups (Figure 1).
When stratifying for sex, we observed that disease duration at cohort entry decreased over time in both males and females, but was always longer in females than in males. Survival was worse in males than in females for all groups (89 vs 95%, Figure 1).
When stratifying for autoantibody, presence of DU at cohort entry decreased significantly over time in ACA+ patients, whereas for ATA+ patients this remained stable. Notably, almost no ACA+ patients had ILD at cohort entry in all groups, while ATA+ patients had a proportion of 25% in 2010-2013 which decreased to 19% in 2018-2021.
Conclusion: Over 10 years of follow-up we observe a decrease in duration between SSc diagnosis and cohort entry, which might indicate more timely diagnosis of SSc. Although trends for less severe organ complications at baseline are observed, no change in 3-year survival is found. Specifically, survival in males is importantly worse than in females, underlining the need for better understanding of disease progression and more effective treatment.
 Table 1. Cohort entry characteristics of included SSc patients from the Leiden CCISS cohort
Table 1. Cohort entry characteristics of included SSc patients from the Leiden CCISS cohort Figure 1. Survival in CCISS cohort.
Figure 1. Survival in CCISS cohort.Disclosures: S. Liem, None; S. Ahmed, Janssen pharamaceuticals; J. Ciaffi, None; L. Beaart-van de Voorde, None; A. Schouffoer, None; J. Geelhoed, None; N. Ajmone Marsan, None; T. Huizinga, None; J. de Vries-Bouwstra, None.

