Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0415: The Association of Early TNF Inhibitor Use with Incident Cardiovascular Events in Ankylosing Spondylitis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Jean Liew, MD, MS
Fellow Physician
Boston University
University of Washington
Boston, MA, United States
Abstract Poster Presenter(s)
Jean Liew1, Timothy Treu2, yojin Park2, Jacqueline Ferguson3, Morgan Rosser2, yuk-Lam Ho2, Susan Heckbert4, Lianne Gensler5, Katherine Liao6 and Maureen Dubreuil7, 1Boston University School of Medicine, Boston, MA, 2VA Boston Healthcare System, Boston, MA, 3Stanford, Palo Alto, CA, 4University of Washington, Seattle, WA, 5Department of Medicine, Division of Rheumatology, University of California San Francisco, San Francisco, CA, 6Brigham and Women's Hospital, Boston, MA, 7Department of Rheumatology, Boston University School of Medicine, Boston, MA
Background/Purpose: The increased cardiovascular (CV) disease burden in ankylosing spondylitis (AS) is established. Whether tumor necrosis factor inhibitor (TNFi) treatment, particularly when started early in the disease course, is cardioprotective is unclear. The aim of our study was to test the association of early TNFi use with incident CV outcomes in an AS cohort.
Methods: We studied a cohort of 17,666 patients with AS within the Veterans Affairs Corporate Data Warehouse between 2002-2019. AS was defined as ≥1 inpatient or ≥2 outpatient ICD codes for AS, at least six weeks apart. Any TNFi use was determined from pharmacy dispense and infusion data. Early TNFi exposure was assessed in the 12-month period following the index date (date of first AS ICD code). The primary outcome was any incident CV disease identified using ICD codes for coronary artery disease, myocardial infarction (MI), or ischemic stroke. Secondary outcomes were MI, venous thromboembolism (VTE), stroke, major adverse cardiovascular events (MACE) including MI, stroke, and CV death, and all-cause mortality. Follow-up started at 12 months after the index date for all subjects until CV event, death, disenrollment, or end of study (December 2019). Those with ≥1 ICD codes for rheumatoid arthritis within one year before AS diagnosis were excluded to improve cohort specificity.
We calculated propensity scores to estimate the probability of a patient starting a TNFi for AS, given the patient characteristics in the 12 months prior to the index date. We fit Cox proportional hazards models with inverse probability weights based on these propensity scores to estimate the risk of each CV outcome with early TNFi initiation compared to non-initiation. Analyses were performed in the overall cohort, and separately in two calendar periods (2002-2010, 2011-2019) to account for secular trends in AS and CV management.
Results: We identified 17,666 individuals with an incident diagnosis of AS (mean age 59.5 years, 91.2% male, 79.8% white), of whom 2,321 were early TNFi initiators and 15,345 were non-TNFi initiators (Table). Mean follow-up was 6.0±4.4 years. TNFi initiators were younger with a lower prevalence of hypertension and diabetes compared to non-TNFi initiators. During follow-up 351 (15.1%) TNFi initiators had incident CVD compared to 3020 (19.7%) non- initiators.
In the propensity score analysis, early TNFi initiation was associated with higher risk of incident CVD (HR 1.17, 95% CI 1.01-1.35), stroke (HR 1.24, 95% CI 1.03-1.50), and MACE (HR 1.22, 95% CI 1.03-1.45) in the cohort overall (Figure). These associations were also demonstrated in calendar period 2011-2019.
Conclusion: In this AS cohort, subjects who initiated TNFi early in their disease course compared to no initiation had a higher risk of incident CVD, and risk of stroke and MACE individually. Although this may represent a true effect, residual confounding by indication cannot be excluded.
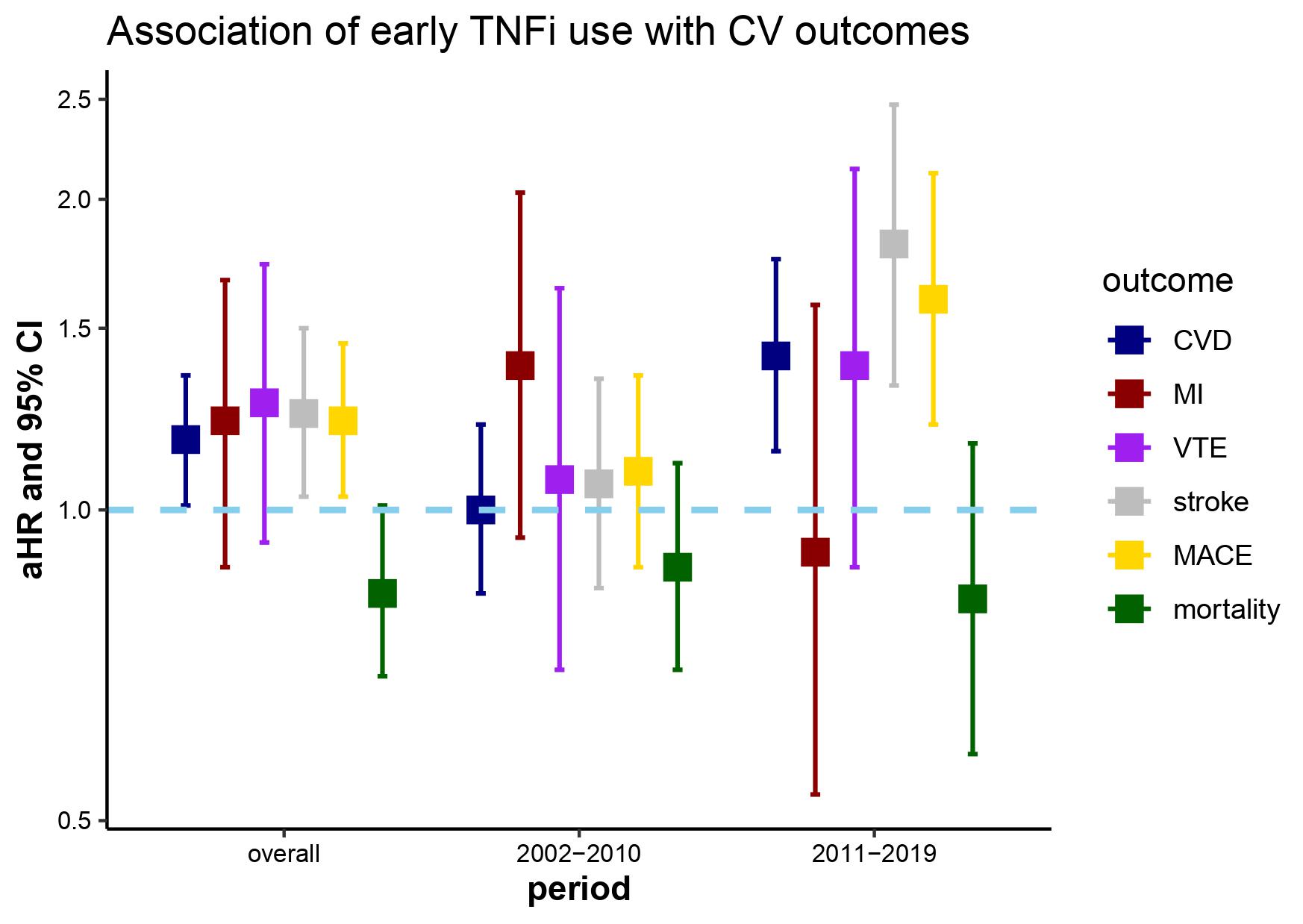 Title: Adjusted hazard ratios for primary and secondary outcomes and early TNFi initiation with among 17,666 individuals with AS between 2002-2019 within the Veterans Affairs Corporate Data Warehouse
Title: Adjusted hazard ratios for primary and secondary outcomes and early TNFi initiation with among 17,666 individuals with AS between 2002-2019 within the Veterans Affairs Corporate Data Warehouse
Figure caption: Adjusted HR and 95% CI are presented for all outcomes in the cohort overall and stratified by calendar periods (2002-2010, 2011-2019). Cox proportional hazards models with inverse probability weighting based on the propensity of TNFi initiation within 12 months following the index date given demographics, comorbidities (hypertension, chronic kidney disease, prior coronary heart disease, prior stroke), NSAID use, glucocorticoid use, non-TNFi disease modifying antirheumatic drug use, spinal imaging orders, physical therapy referrals, referrals to pain clinic/acupuncture/chiropractor, primary care visits, rheumatology visits, and emergency department/urgent care visits in the 12 months prior to the index date. Models additionally adjusted for age as a quadratic term, DMARD use, and rheumatology visits. Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; CVD, cardiovascular disease; MI, myocardial infarction; VTE, venous thromboembolism; MACE, major adverse cardiac events
Disclosures: J. Liew, None; T. Treu, None; y. Park, None; J. Ferguson, None; M. Rosser, None; y. Ho, None; S. Heckbert, None; L. Gensler, Novartis, Pfizer Inc, UCB Pharma, AbbVie, Eli Lilly, Janssen, Gilead, Moonlake; K. Liao, None; M. Dubreuil, Amgen, UCB Pharma, Pfizer.
Background/Purpose: The increased cardiovascular (CV) disease burden in ankylosing spondylitis (AS) is established. Whether tumor necrosis factor inhibitor (TNFi) treatment, particularly when started early in the disease course, is cardioprotective is unclear. The aim of our study was to test the association of early TNFi use with incident CV outcomes in an AS cohort.
Methods: We studied a cohort of 17,666 patients with AS within the Veterans Affairs Corporate Data Warehouse between 2002-2019. AS was defined as ≥1 inpatient or ≥2 outpatient ICD codes for AS, at least six weeks apart. Any TNFi use was determined from pharmacy dispense and infusion data. Early TNFi exposure was assessed in the 12-month period following the index date (date of first AS ICD code). The primary outcome was any incident CV disease identified using ICD codes for coronary artery disease, myocardial infarction (MI), or ischemic stroke. Secondary outcomes were MI, venous thromboembolism (VTE), stroke, major adverse cardiovascular events (MACE) including MI, stroke, and CV death, and all-cause mortality. Follow-up started at 12 months after the index date for all subjects until CV event, death, disenrollment, or end of study (December 2019). Those with ≥1 ICD codes for rheumatoid arthritis within one year before AS diagnosis were excluded to improve cohort specificity.
We calculated propensity scores to estimate the probability of a patient starting a TNFi for AS, given the patient characteristics in the 12 months prior to the index date. We fit Cox proportional hazards models with inverse probability weights based on these propensity scores to estimate the risk of each CV outcome with early TNFi initiation compared to non-initiation. Analyses were performed in the overall cohort, and separately in two calendar periods (2002-2010, 2011-2019) to account for secular trends in AS and CV management.
Results: We identified 17,666 individuals with an incident diagnosis of AS (mean age 59.5 years, 91.2% male, 79.8% white), of whom 2,321 were early TNFi initiators and 15,345 were non-TNFi initiators (Table). Mean follow-up was 6.0±4.4 years. TNFi initiators were younger with a lower prevalence of hypertension and diabetes compared to non-TNFi initiators. During follow-up 351 (15.1%) TNFi initiators had incident CVD compared to 3020 (19.7%) non- initiators.
In the propensity score analysis, early TNFi initiation was associated with higher risk of incident CVD (HR 1.17, 95% CI 1.01-1.35), stroke (HR 1.24, 95% CI 1.03-1.50), and MACE (HR 1.22, 95% CI 1.03-1.45) in the cohort overall (Figure). These associations were also demonstrated in calendar period 2011-2019.
Conclusion: In this AS cohort, subjects who initiated TNFi early in their disease course compared to no initiation had a higher risk of incident CVD, and risk of stroke and MACE individually. Although this may represent a true effect, residual confounding by indication cannot be excluded.
 Title: Adjusted hazard ratios for primary and secondary outcomes and early TNFi initiation with among 17,666 individuals with AS between 2002-2019 within the Veterans Affairs Corporate Data Warehouse
Title: Adjusted hazard ratios for primary and secondary outcomes and early TNFi initiation with among 17,666 individuals with AS between 2002-2019 within the Veterans Affairs Corporate Data WarehouseFigure caption: Adjusted HR and 95% CI are presented for all outcomes in the cohort overall and stratified by calendar periods (2002-2010, 2011-2019). Cox proportional hazards models with inverse probability weighting based on the propensity of TNFi initiation within 12 months following the index date given demographics, comorbidities (hypertension, chronic kidney disease, prior coronary heart disease, prior stroke), NSAID use, glucocorticoid use, non-TNFi disease modifying antirheumatic drug use, spinal imaging orders, physical therapy referrals, referrals to pain clinic/acupuncture/chiropractor, primary care visits, rheumatology visits, and emergency department/urgent care visits in the 12 months prior to the index date. Models additionally adjusted for age as a quadratic term, DMARD use, and rheumatology visits. Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; CVD, cardiovascular disease; MI, myocardial infarction; VTE, venous thromboembolism; MACE, major adverse cardiac events
Disclosures: J. Liew, None; T. Treu, None; y. Park, None; J. Ferguson, None; M. Rosser, None; y. Ho, None; S. Heckbert, None; L. Gensler, Novartis, Pfizer Inc, UCB Pharma, AbbVie, Eli Lilly, Janssen, Gilead, Moonlake; K. Liao, None; M. Dubreuil, Amgen, UCB Pharma, Pfizer.

