Back
Poster Session D
Session: (2154–2178) Systemic Sclerosis and Related Disorders – Clinical Poster III
2161: Alterations of Nutritional Status of Scleroderma Patients and Associations with Disease-specific Features
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Sabína Oreská, MD
Institute of Rheumatology
Prague, Czech Republic
Abstract Poster Presenter(s)
Sabína Oreská1, Hana Storkanova1, Maja Spiritovic2, Barbora Hermankova2, Michal Vrablík3, Karel Pavelka4, Jiří Vencovský5, Ladislav Šenolt5, Radim Becvar1 and Michal Tomcik1, 1Institute of Rheumatology, Department of Rheumatology, 1st Faculty of Medicine, Charles University, Prague, Czech Republic, Prague, Czech Republic, 2Institute of Rheumatology, Department of Physiotherapy, Faculty of Physical Education and Sport, Charles University, Prague, Czech Republic, Prague, Czech Republic, 33rd Department of Internal Medicine, General University Hospital and 1st Faculty of Medicine, Charles University, Prague, Czech Republic, Prague, Czech Republic, 4Institute of Rheumatology, Department of Rheumatology, 1st Faculty of Medicine, Charles University, Prague, Czech Republic, Praha, Czech Republic, 5Institute of Rheumatology and Department of Rheumatology, First Faculty of Medicine, Charles University, Prague, Czech Republic
Background/Purpose: Systemic sclerosis (SSc) is characterized by skin and organ involvement and chronic disease course. In particular, involvement of the gastrointestinal tract and systemic inflammation can have a negative impact on nutritional status. The aim of this study was to assess the differences in the nutritional status of SSc patients and healthy controls (HC) and the association with disease-specific features.
Methods: 100 patients with SSc (85 females; mean age 55.5; disease duration 4.0 years; lcSSc 59 / dcSSc 41) and 100 age-/sex-matched HC (85 females, mean age 55.3) without rheumatic diseases were included. Patients with SSc fulfilled the 2013 ACR/EULAR criteria. Levels of selected nutrition parameters were measured in sera drawn after 8 hours of fasting by routine analytic methods. In SSc patients, disease activity was evaluated by the ESSG composite index, skin involvement by the modified Rodnan skin score (mRSS), and organ involvement and current treatment were recorded.
Results: Differences in disease activity and skin involvement were observed between the two subtypes of SSc, whereas comorbidities, internal organ involvement, and treatment were statisticaly comparable. Serum levels of proteins (total protein, albumin, prealbumin, transferrin), minerals (Fe, Mg, Zn), and vitamins (calcidiol, calcitriol) were decreased (p=0.060 for total protein, p< 0.05 for the rest). On the other hand, orosomucoid (an inflammatory marker) and C-peptide were increased in SSc compared to HC (p< 0.05). Patients with dsSSc had a more unfavorable decrease in minerals (Fe, Mg) than lcSSc when compared to the corresponding age-/sex-matched HC. Among the individual SSc subsets, lcSSc had a trend to higher albumin, Mg, and Zn levels (p< 0.10) and significantly higher albumin levels (p=0.033) compared to dcSSc. The remaining nutritional markers were comparable between lcSSc and dcSSc. High disease activity, increased levels of CRP or ESR, worse skin involvement (higher mRSS), and lower BMI were associated with adverse changes in nutritional markers, especially decreased albumin, prealbumin, transferrin, minerals, and vitamins. Other factors significantly associated with nutrition parameters were the current dose of glucocorticoids, disease duration, lung involvement, and age (table 1).
Conclusion: We have observed significant alterations in nutritional parameters in our SSc patients compared to age-/sex-matched healthy individuals. These changes were associated predominantly with disease activity and skin involvement. Patients with dcSSc tended to have worse disease activity and more pronounced alterations of nutritional status compared to lsSSc patients.
Acknowledgment: Supported by AZV NV18-01-00161A, MHCR-00023728, and SVV-260523.
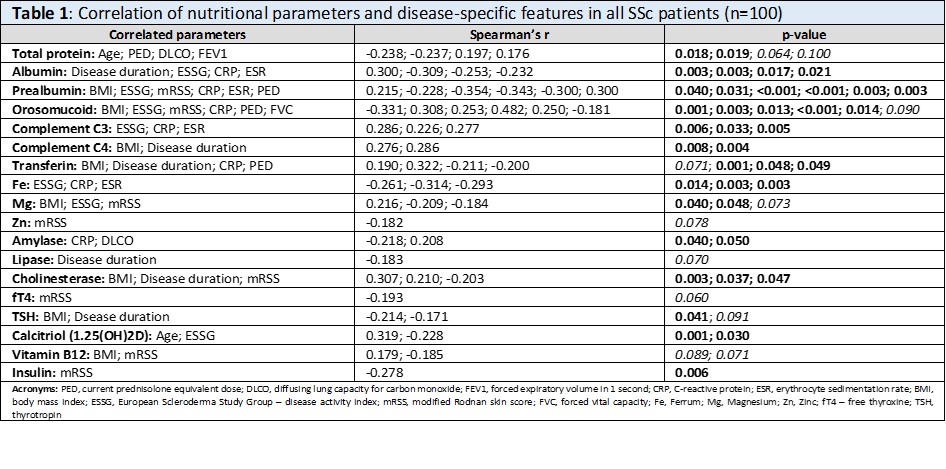 Table 1: Correlation of nutritional parameters and disease-specific features in all SSc patients (n=100)
Table 1: Correlation of nutritional parameters and disease-specific features in all SSc patients (n=100)
Disclosures: S. Oreská, None; H. Storkanova, None; M. Spiritovic, None; B. Hermankova, None; M. Vrablík, None; K. Pavelka, MSD, Pfizer, Roche, Eli Lilly, Medac, UCB, SOBI, Biogen, Sandoz, Viatris; J. Vencovský, Abbvie, Biogen, Boehringer, Eli Lilly, Gilead, Kezar, Merck, Novartis, Octapharma, Pfizer, Takeda, UCB, Werfen, Argenx; L. Šenolt, None; R. Becvar, None; M. Tomcik, None.
Background/Purpose: Systemic sclerosis (SSc) is characterized by skin and organ involvement and chronic disease course. In particular, involvement of the gastrointestinal tract and systemic inflammation can have a negative impact on nutritional status. The aim of this study was to assess the differences in the nutritional status of SSc patients and healthy controls (HC) and the association with disease-specific features.
Methods: 100 patients with SSc (85 females; mean age 55.5; disease duration 4.0 years; lcSSc 59 / dcSSc 41) and 100 age-/sex-matched HC (85 females, mean age 55.3) without rheumatic diseases were included. Patients with SSc fulfilled the 2013 ACR/EULAR criteria. Levels of selected nutrition parameters were measured in sera drawn after 8 hours of fasting by routine analytic methods. In SSc patients, disease activity was evaluated by the ESSG composite index, skin involvement by the modified Rodnan skin score (mRSS), and organ involvement and current treatment were recorded.
Results: Differences in disease activity and skin involvement were observed between the two subtypes of SSc, whereas comorbidities, internal organ involvement, and treatment were statisticaly comparable. Serum levels of proteins (total protein, albumin, prealbumin, transferrin), minerals (Fe, Mg, Zn), and vitamins (calcidiol, calcitriol) were decreased (p=0.060 for total protein, p< 0.05 for the rest). On the other hand, orosomucoid (an inflammatory marker) and C-peptide were increased in SSc compared to HC (p< 0.05). Patients with dsSSc had a more unfavorable decrease in minerals (Fe, Mg) than lcSSc when compared to the corresponding age-/sex-matched HC. Among the individual SSc subsets, lcSSc had a trend to higher albumin, Mg, and Zn levels (p< 0.10) and significantly higher albumin levels (p=0.033) compared to dcSSc. The remaining nutritional markers were comparable between lcSSc and dcSSc. High disease activity, increased levels of CRP or ESR, worse skin involvement (higher mRSS), and lower BMI were associated with adverse changes in nutritional markers, especially decreased albumin, prealbumin, transferrin, minerals, and vitamins. Other factors significantly associated with nutrition parameters were the current dose of glucocorticoids, disease duration, lung involvement, and age (table 1).
Conclusion: We have observed significant alterations in nutritional parameters in our SSc patients compared to age-/sex-matched healthy individuals. These changes were associated predominantly with disease activity and skin involvement. Patients with dcSSc tended to have worse disease activity and more pronounced alterations of nutritional status compared to lsSSc patients.
Acknowledgment: Supported by AZV NV18-01-00161A, MHCR-00023728, and SVV-260523.
 Table 1: Correlation of nutritional parameters and disease-specific features in all SSc patients (n=100)
Table 1: Correlation of nutritional parameters and disease-specific features in all SSc patients (n=100)Disclosures: S. Oreská, None; H. Storkanova, None; M. Spiritovic, None; B. Hermankova, None; M. Vrablík, None; K. Pavelka, MSD, Pfizer, Roche, Eli Lilly, Medac, UCB, SOBI, Biogen, Sandoz, Viatris; J. Vencovský, Abbvie, Biogen, Boehringer, Eli Lilly, Gilead, Kezar, Merck, Novartis, Octapharma, Pfizer, Takeda, UCB, Werfen, Argenx; L. Šenolt, None; R. Becvar, None; M. Tomcik, None.

