Back
Poster Session D
Session: (2108–2153) Spondyloarthritis Including PsA – Treatment Poster III: PsA
2130: Apremilast in Bio-Naïve Patients with Early Psoriatic Arthritis: A National, Real-Life, Multicenter, Non-Interventional, Prospective, 52-week Cohort Study
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GK
Gkikas Katsifis, MD, PhD, RhMSUS
Naval Hospital of Athens
ATHENS, Greece
Abstract Poster Presenter(s)
Petros P. Sfikakis1, Dimitrios Vassilopoulos2, Gkikas Katsifis3, Georgios Vosvotekas4, Theodoros Dimitroulas5, Argyro Repa6, Grigorios Sakellariou7, THEODORA SIMOPOULOU8, Athanasios Georgountzos9, Andreas Bounas10, Panagiotis Georgiou11, Evangelia Mole12, Evangelia Kataxaki13, Stamatis Nick Liossis14, Evangelos Theodorou15, Christina Antoniadou16, EVANGELOS THEOTIKOS17, PANAYIOTIS VLACHOYIANNOPOULOS18, THEODORA MARKATSELI19, Angeliki Kekki20, NIKOLAOS ANTONAKOPOULOS21 and Dimitrios Boumpas22, 1Joint Academic Rheumatology Program, National and Kapodistrian University of Athens, School of Medicine, Athens, Greece, 2Clinical Immunology-Rheumatology Unit, 2nd Department of Internal Medicine and Laboratory, National and Kapodistrian University of Athens, School of Medicine, Hippokration General Hospital, Athens, Greece, 3Naval Hospital of Athens, Athens, Greece, 4Euromedica General Clinic of Thessaloniki, Thessaloniki, Greece, Thessaloniki, Greece, 54th Department of Internal medicine, Hippokration Hospital, School of medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece, Thessaloniki, Greece, 6Rheumatology, Clinical Immunology and Allergy, University of Crete School of Medicine, Heraklion, Crete, Greece, 7Department of Rheumatology, 424 General Army Hospital, Thessaloniki, Greece, Thessaloniki, Greece, 8Clinic of Rheumatology and Clinical Immunology, University Hospital of Larissa, Larissa, Greece, Larissa, Larisa, Greece, 9General Hospital of Athens G. Gennimatas, Athens, Greece, 10Olympion private General Clinic of Patras, Patras, Greece;, Patra, Akhaia, Greece, 11Rheumatology Unit, Agios Andreas Hospital, Patras, Greece, PATRA, Akhaia, Greece, 12Department of Rheumatology, KAT General Hospital of Attica, Athens, Greece, 13Rheumatology Unit, Thriasio General Hospital of Elefsina, Magoula, Greece, 14Division of Rheumatology, Department of Internal Medicine, Patras University Hospital, University of Patras Medical School, Patras, Greece, Patras, Akhaia, Greece, 15Rheumatology Clinic 251 Hellenic Air Force Hospital, Athens, Greece, 16First Department of Internal Medicine, University Hospital of Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece, 17Rheumatology Department, Asklepieion Voulas General Hospital, Athens, Greece, 18Department of Pathophysiology, Medical School, National and Kapodistrian University of Athens, Athens, Greece, 19Rheumatology Clinic, Department of Internal Medicine, Medical School, University of Ioannina, Ioannina, Greece, 20Genesis Pharma SA, Halandri, Greece, 21Genesis Pharma SA, Chalandri, Greece, 224th Department of Internal Medicine, "Attikon" University Hospital, Athens, Joint Academic Rheumatology Program, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Background/Purpose: Apremilast is a targeted synthetic disease-modifying anti-rheumatic drug (tsDMARD) approved for psoriatic arthritis (PsA). Few clinical studies assessing treatment regimens in PsA have focused on patients with early disease. The current APROACH study investigated the impact of apremilast in biologic-naïve patients with early peripheral PsA.
Methods: This was a nationwide, non-interventional, multicenter, 52-week prospective cohort study in biologic-naïve patients with early (start of first ever treatment < 12 months prior to recruitment), peripheral PsA, who started apremilast after an inadequate response/intolerance to a initial conventional synthetic (cs)DMARD treatment (< 12 months total treatment duration). Data were collected through routine clinical assessment, patient self-report, and medical records at enrollment, and at 16, 24, and 52 weeks after apremilast initiation. Non-responder imputation was applied for missing data.
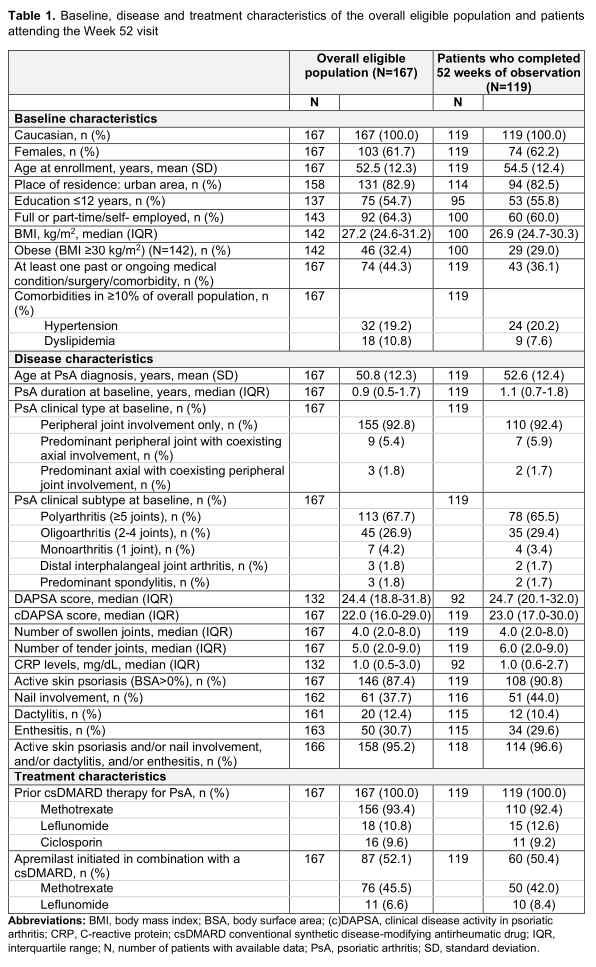
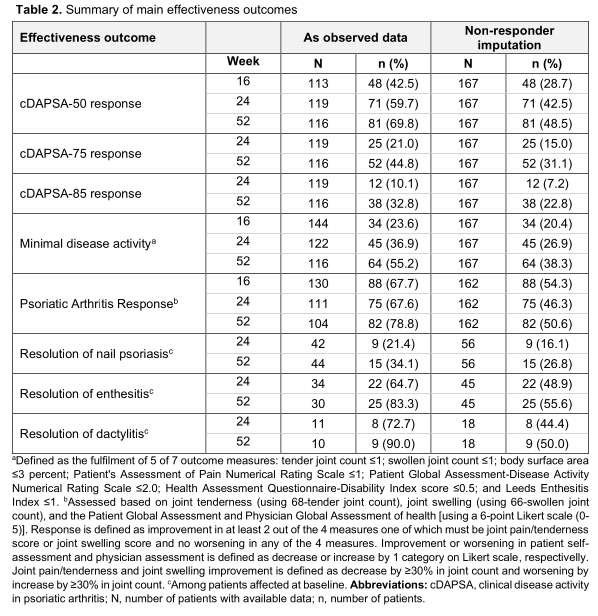
Results: A total of 167 eligible consecutively enrolled patients (mean age: 52.5 years) with a median PsA duration of 11.2 months were enrolled between 15-Apr-2019 and 06-Jul-2020. All patients had previously received (cs)DMARDs and 52.1% received apremilast in combination with a csDMARD. The median [interquartile range (IQR)] exposure to apremilast during the study observation period was 12.0 (8.2-12.2) months; 119 patients attended the Week 52 visit, while 48 prematurely withdrew from the study, due to apremilast discontinuation (34/48), loss to follow-up (12/48), and COVID-19 restrictions (2/48). Table 1 shows the characteristics of the overall population and patients completing 52 weeks of observation. At baseline, the median (IQR) clinical disease activity in psoriatic arthritis (cDAPSA) score was 22.0 (16.0-29.0), with 86.8% of the patients having at least moderate (29.3% high) disease activity; 87.4% of evaluable patients had skin psoriasis, 37.7% nail psoriasis, 30.7% enthesitis, and 12.4% dactylitis. At 16, 24, and 52 weeks, the median cDAPSA score decreased to 14, 10, and 6, respectively. Among evaluable patients 9%, 15.1%, and 37.9% achieved remission (cDAPSA< 4) while 40.7%, 49.6%, and 40.5% attained low disease activity (cDAPSA< 14) at the respected time points. Complete resolution of skin psoriasis was achieved by 26.7%, nail psoriasis by 26.8%, enthesitis by 55.6%, and dactylitis by 50.0% of patients. The adverse drug reaction rate was 13.8%, including only 1 serious adverse event (major depression, that resolved by the end of the observation period). 11 patients (6.6%) discontinued apremilast due to adverse events.
Conclusion: The APROACH real-life study shows that ~40% of patients (bio-naïve, csDMARD-non-responders/intolerant) with early PsA achieved remission of their peripheral arthritis after 52 weeks of apremilast treatment. The rest of the different manifestations of the psoriatic disease also improved significantly while the drug's safety profile was consistent with previous reports. These real-life data highlight the value of early therapy with apremilast in biologic-naïve patients with PsA who have not responded to (cs)DMARDs.
Disclosures: P. Sfikakis, Pfizer, AbbVie/Abbott, Novartis, Amgen, Janssen, Boehringer-Ingelheim, Celgene, Eli Lilly; D. Vassilopoulos, Genesis Pharma SA; G. Katsifis, AbbVie/Abbott, Aenorasis, Amgen, Genesis Pharma SA, Celgene, Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, Sobi, UCB; G. Vosvotekas, Genesis Pharma SA, Genesis Pharma SA; T. Dimitroulas, Genesis Pharma SA, AbbVie/Abbott, Eli Lilly, Novartis, Pfizer, UCB, Elpen, Boehringer-Ingelheim, Mylan, Amgen, Demo, Merck/MSD, Aenorasis, Faran; A. Repa, Boehringer-Ingelheim, Merck/MSD, Elpen, AbbVie/Abbott; G. Sakellariou, Genesis Pharma SA, Genesis Pharma SA, AbbVie/Abbott, Merck/MSD, UCB; T. SIMOPOULOU, Elpen, Boehringer-Ingelheim, Genesis Pharma SA, Genesis Pharma SA, Janssen, Novartis, Pfizer, Pfizer; A. Georgountzos, AbbVie/Abbott, AbbVie/Abbott, Genesis Pharma SA, Janssen, Mylan, UCB, Boehringer-Ingelheim, Roche; A. Bounas, AbbVie/Abbott, Aenorasis, Amgen, Bausch Health, Faran, Genesis Pharma SA, GlaxoSmithKlein(GSK), Janssen, Merck/MSD, Novartis, Pfizer, UCB, AbbVie/Abbott, Amgen, Genesis Pharma SA, Merck/MSD, Novartis, Pfizer; P. Georgiou, UCB, Genesis Pharma SA, AbbVie/Abbott, Mylan, Aenorasis, Janssen; E. Mole, AbbVie/Abbott, Genesis Pharma SA, Janssen, Merck/MSD, Pfizer, Roche, UCB; E. Kataxaki, Genesis Pharma SA, Mylan; S. Liossis, None; E. Theodorou, Amgen, AbbVie/Abbott, Aenorasis, Faran, GlaxoSmithKlein(GSK); C. Antoniadou, None; E. THEOTIKOS, None; P. VLACHOYIANNOPOULOS, None; T. MARKATSELI, None; A. Kekki, Genesis Pharma SA; N. ANTONAKOPOULOS, Genesis Pharma SA; D. Boumpas, None.
Background/Purpose: Apremilast is a targeted synthetic disease-modifying anti-rheumatic drug (tsDMARD) approved for psoriatic arthritis (PsA). Few clinical studies assessing treatment regimens in PsA have focused on patients with early disease. The current APROACH study investigated the impact of apremilast in biologic-naïve patients with early peripheral PsA.
Methods: This was a nationwide, non-interventional, multicenter, 52-week prospective cohort study in biologic-naïve patients with early (start of first ever treatment < 12 months prior to recruitment), peripheral PsA, who started apremilast after an inadequate response/intolerance to a initial conventional synthetic (cs)DMARD treatment (< 12 months total treatment duration). Data were collected through routine clinical assessment, patient self-report, and medical records at enrollment, and at 16, 24, and 52 weeks after apremilast initiation. Non-responder imputation was applied for missing data.
Results: A total of 167 eligible consecutively enrolled patients (mean age: 52.5 years) with a median PsA duration of 11.2 months were enrolled between 15-Apr-2019 and 06-Jul-2020. All patients had previously received (cs)DMARDs and 52.1% received apremilast in combination with a csDMARD. The median [interquartile range (IQR)] exposure to apremilast during the study observation period was 12.0 (8.2-12.2) months; 119 patients attended the Week 52 visit, while 48 prematurely withdrew from the study, due to apremilast discontinuation (34/48), loss to follow-up (12/48), and COVID-19 restrictions (2/48). Table 1 shows the characteristics of the overall population and patients completing 52 weeks of observation. At baseline, the median (IQR) clinical disease activity in psoriatic arthritis (cDAPSA) score was 22.0 (16.0-29.0), with 86.8% of the patients having at least moderate (29.3% high) disease activity; 87.4% of evaluable patients had skin psoriasis, 37.7% nail psoriasis, 30.7% enthesitis, and 12.4% dactylitis. At 16, 24, and 52 weeks, the median cDAPSA score decreased to 14, 10, and 6, respectively. Among evaluable patients 9%, 15.1%, and 37.9% achieved remission (cDAPSA< 4) while 40.7%, 49.6%, and 40.5% attained low disease activity (cDAPSA< 14) at the respected time points. Complete resolution of skin psoriasis was achieved by 26.7%, nail psoriasis by 26.8%, enthesitis by 55.6%, and dactylitis by 50.0% of patients. The adverse drug reaction rate was 13.8%, including only 1 serious adverse event (major depression, that resolved by the end of the observation period). 11 patients (6.6%) discontinued apremilast due to adverse events.
Conclusion: The APROACH real-life study shows that ~40% of patients (bio-naïve, csDMARD-non-responders/intolerant) with early PsA achieved remission of their peripheral arthritis after 52 weeks of apremilast treatment. The rest of the different manifestations of the psoriatic disease also improved significantly while the drug's safety profile was consistent with previous reports. These real-life data highlight the value of early therapy with apremilast in biologic-naïve patients with PsA who have not responded to (cs)DMARDs.


Disclosures: P. Sfikakis, Pfizer, AbbVie/Abbott, Novartis, Amgen, Janssen, Boehringer-Ingelheim, Celgene, Eli Lilly; D. Vassilopoulos, Genesis Pharma SA; G. Katsifis, AbbVie/Abbott, Aenorasis, Amgen, Genesis Pharma SA, Celgene, Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, Sobi, UCB; G. Vosvotekas, Genesis Pharma SA, Genesis Pharma SA; T. Dimitroulas, Genesis Pharma SA, AbbVie/Abbott, Eli Lilly, Novartis, Pfizer, UCB, Elpen, Boehringer-Ingelheim, Mylan, Amgen, Demo, Merck/MSD, Aenorasis, Faran; A. Repa, Boehringer-Ingelheim, Merck/MSD, Elpen, AbbVie/Abbott; G. Sakellariou, Genesis Pharma SA, Genesis Pharma SA, AbbVie/Abbott, Merck/MSD, UCB; T. SIMOPOULOU, Elpen, Boehringer-Ingelheim, Genesis Pharma SA, Genesis Pharma SA, Janssen, Novartis, Pfizer, Pfizer; A. Georgountzos, AbbVie/Abbott, AbbVie/Abbott, Genesis Pharma SA, Janssen, Mylan, UCB, Boehringer-Ingelheim, Roche; A. Bounas, AbbVie/Abbott, Aenorasis, Amgen, Bausch Health, Faran, Genesis Pharma SA, GlaxoSmithKlein(GSK), Janssen, Merck/MSD, Novartis, Pfizer, UCB, AbbVie/Abbott, Amgen, Genesis Pharma SA, Merck/MSD, Novartis, Pfizer; P. Georgiou, UCB, Genesis Pharma SA, AbbVie/Abbott, Mylan, Aenorasis, Janssen; E. Mole, AbbVie/Abbott, Genesis Pharma SA, Janssen, Merck/MSD, Pfizer, Roche, UCB; E. Kataxaki, Genesis Pharma SA, Mylan; S. Liossis, None; E. Theodorou, Amgen, AbbVie/Abbott, Aenorasis, Faran, GlaxoSmithKlein(GSK); C. Antoniadou, None; E. THEOTIKOS, None; P. VLACHOYIANNOPOULOS, None; T. MARKATSELI, None; A. Kekki, Genesis Pharma SA; N. ANTONAKOPOULOS, Genesis Pharma SA; D. Boumpas, None.

