Back
Poster Session D
Session: (2108–2153) Spondyloarthritis Including PsA – Treatment Poster III: PsA
2122: Bimekizumab Treatment Improves Health-Related Quality of Life in Biologic DMARD-Naïve and TNFi-IR Patients with Active PsA: Pooled 16-Week Results from Two Phase 3 Randomized, Placebo-Controlled Studies
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.jpg)
Dafna Gladman, MD, FRCPC
University of Toronto, Toronto Western Hospital
Toronto, ON, Canada
Abstract Poster Presenter(s)
Dafna Gladman1, Lars Erik2, Diamant Thaci3, Paolo Gisondi4, Laure Gossec5, M. Elaine Husni6, Alice Gottlieb7, Hiroaki Dobashi8, Barbara Ink9, Deepak Assudani9, Rajan Bajracharya9, Jason Coarse10, Jérémy Lambert11 and William Tillett12, 1Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 2Bispebjerg-Frederiksberg Hospital, Vedbæk, Denmark, 3Institute and Comprehensive Center for Inflammation Medicine, University Hospital of Lübeck, Lübeck, Germany, 4Dermatology and Venereology, Department of Medicine, Università di Verona, Verona, Italy, Verona, 5Sorbonne Université, Paris, France, 6Cleveland Clinic, Cleveland, OH, 7Department of Dermatology, The Icahn School of Medicine at Mount Sinai, New York, NY, 8Division of Hematology, Rheumatology and Respiratory Medicine, Department of Internal Medicine, Faculty of Medicine, Kagawa, Japan, 9UCB Pharma, Slough, United Kingdom, 10UCB Pharma, Raleigh, NC, USA, Raleigh, NC, 11UCB Pharma, Colombes, France, Irigny, France, 12Royal National Hospital for Rheumatic Diseases, Bath, United Kingdom
Background/Purpose: PsA imparts a substantial burden on patient health-related quality of life (HRQoL).1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has shown efficacy and tolerability up to 16 weeks (wks) in patients (pts) with active PsA in the phase 3 BE OPTIMAL and BE COMPLETE studies.2,3
This analysis aimed to assess impact of BKZ treatment vs placebo (PBO) on HRQoL in pts with active PsA that are biologic DMARD (bDMARD)-naïve or had inadequate response to TNF inhibitors (TNFi-IR).
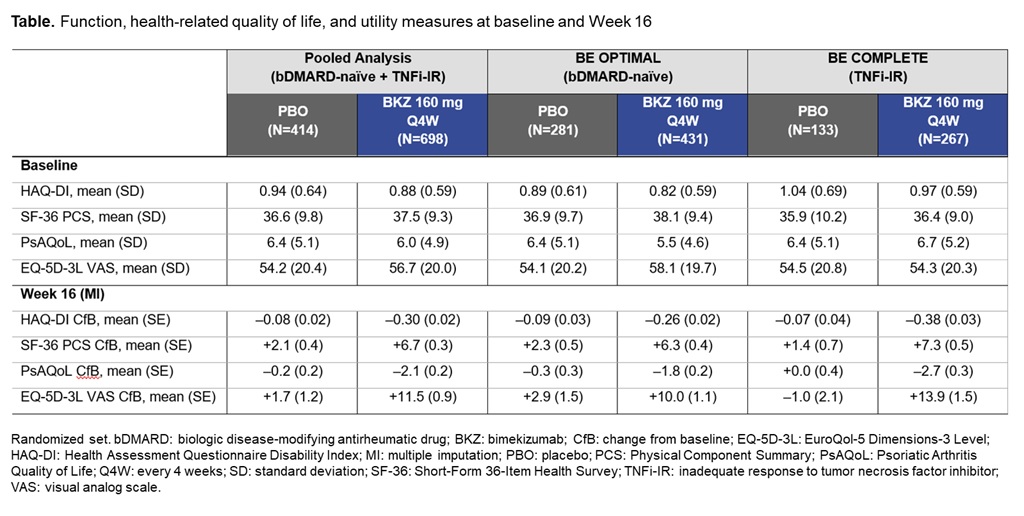
Methods: BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) are phase 3 studies of BKZ in bDMARD-naïve or TNFi-IR pts with PsA, respectively. In BE OPTIMAL, 852 pts were randomized 3:2:1 to subcutaneous (sc) BKZ 160 mg every 4 wks (Q4W), placebo (PBO), or a reference arm (sc adalimumab 40 mg every 2 wks); in BE COMPLETE, 400 pts were randomized 2:1 to sc BKZ 160 mg Q4W or PBO. Primary endpoint in both studies was ACR50 at Wk 16. Measures of function, HRQoL, and utility (pt preference attached to health status)4 included, but were not limited to, Health Assessment Questionnaire Disability Index (HAQ‑DI) (pts with minimal clinically important difference [MCID, ≥0.35 reduction from baseline (BL)], normative state [score ≤0.5],5,6 and change from BL [CfB]), Short-Form 36-Item Health Survey (SF‑36) Physical Component Summary (PCS), PsAQoL, and EuroQol 5-Dimensions 3-Level visual analog scale (EQ-5D-3L VAS). Non‑responder/multiple imputation (NRI, MI) were used for missing binary/continuous variables.
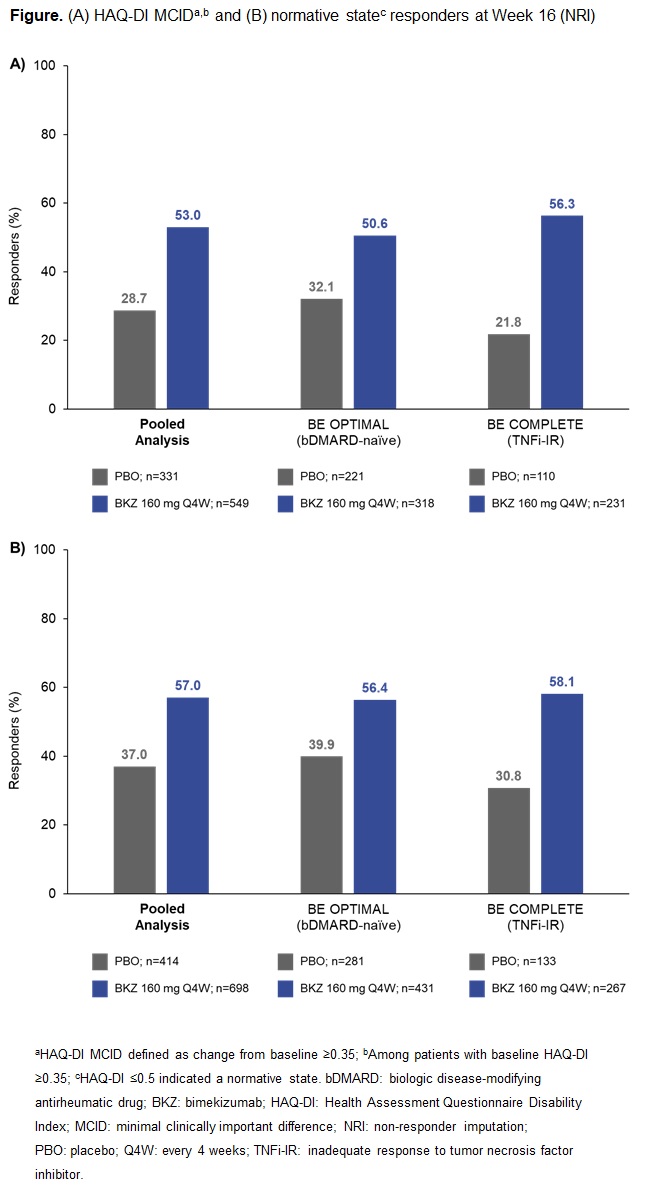
Results: A total of 1,073/1,112 (96.5%) pts randomized to BKZ or PBO completed Wk 16 in BE OPTIMAL and BE COMPLETE. Patient demographics and BL function, HRQoL, and utility scores were generally comparable between PBO and BKZ arms within and across trials (Table).2,3 At Wk 16 (pooled), BKZ-treated pts showed greater improvements in physical function vs PBO‑treated pts, including for key outcomes HAQ-DI MCID (BKZ: 53.0%, PBO: 28.7%) and HAQ-DI ≤0.5 (BKZ: 57.0%, PBO: 37.0%) (Figure), as well as for CfB in norm-based SF‑36 PCS (Table). BKZ-treated pts also showed greater improvements at Wk 16 in HRQoL (PsAQoL CfB; BKZ: –2.1, PBO: –0.2) and utility (EQ-5D-3L VAS CfB; BKZ: +11.5, PBO: +1.7) (Table). BKZ results were similar between bDMARD-naïve and TNFi-IR pts in BE OPTIMAL and BE COMPLETE, respectively (Table).
Conclusion: BKZ treatment resulted in clinically meaningful improvements in measures of HRQoL, physical function, and utility compared with PBO in pts with active PsA. Across measures, results were consistent between bDMARD-naïve and TNFi-IR pts.
References: 1. Gudu T. Expert Rev Clin Immunol 2018;14(5):405–17; 2. McInnes IB. Ann Rheum Dis 2022;81:206–7; 3. Merola JF. Ann Rheum Dis 2022;81:167–8; 4. Bakker CH. Patient Educ Couns 1993;20(2–3):145–52; 5. Krishnan E. Arthritis Rheum 2004;50(3):953–90; 6. Keystone EC. J Rheumatol 2013;40(9):1487–97.
Disclosures: D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; L. Erik, AbbVie/Abbott, Amgen, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Gilead, Janssen, Merck/MSD, Novartis, Novo Nordisk, Pfizer, UCB Pharma; D. Thaci, AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi-Genzyme, UCB Pharma, Celltrion, Morphosis, Sun Pharma; P. Gisondi, AbbVie, Abiogen, Almirall, Celgene, Eli Lilly, Janssen, LEO Pharma, Merck/MSD, Novartis, Otsuka, Pfizer, Pierre Fabre, Sanofi, UCB Pharma; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; A. Gottlieb, Amgen, AnaptsysBio, Avortres Therapeutics, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Sun Pharmaceuticals Industries, UCB Pharma, Xbiotech, Ortho; H. Dobashi, BMS, Chugai; B. Ink, UCB Pharma, GlaxoSmithKlein(GSK); D. Assudani, UCB Pharma; R. Bajracharya, UCB Pharma; J. Coarse, UCB Pharma; J. Lambert, UCB Pharma; W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB.
Background/Purpose: PsA imparts a substantial burden on patient health-related quality of life (HRQoL).1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has shown efficacy and tolerability up to 16 weeks (wks) in patients (pts) with active PsA in the phase 3 BE OPTIMAL and BE COMPLETE studies.2,3
This analysis aimed to assess impact of BKZ treatment vs placebo (PBO) on HRQoL in pts with active PsA that are biologic DMARD (bDMARD)-naïve or had inadequate response to TNF inhibitors (TNFi-IR).
Methods: BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) are phase 3 studies of BKZ in bDMARD-naïve or TNFi-IR pts with PsA, respectively. In BE OPTIMAL, 852 pts were randomized 3:2:1 to subcutaneous (sc) BKZ 160 mg every 4 wks (Q4W), placebo (PBO), or a reference arm (sc adalimumab 40 mg every 2 wks); in BE COMPLETE, 400 pts were randomized 2:1 to sc BKZ 160 mg Q4W or PBO. Primary endpoint in both studies was ACR50 at Wk 16. Measures of function, HRQoL, and utility (pt preference attached to health status)4 included, but were not limited to, Health Assessment Questionnaire Disability Index (HAQ‑DI) (pts with minimal clinically important difference [MCID, ≥0.35 reduction from baseline (BL)], normative state [score ≤0.5],5,6 and change from BL [CfB]), Short-Form 36-Item Health Survey (SF‑36) Physical Component Summary (PCS), PsAQoL, and EuroQol 5-Dimensions 3-Level visual analog scale (EQ-5D-3L VAS). Non‑responder/multiple imputation (NRI, MI) were used for missing binary/continuous variables.
Results: A total of 1,073/1,112 (96.5%) pts randomized to BKZ or PBO completed Wk 16 in BE OPTIMAL and BE COMPLETE. Patient demographics and BL function, HRQoL, and utility scores were generally comparable between PBO and BKZ arms within and across trials (Table).2,3 At Wk 16 (pooled), BKZ-treated pts showed greater improvements in physical function vs PBO‑treated pts, including for key outcomes HAQ-DI MCID (BKZ: 53.0%, PBO: 28.7%) and HAQ-DI ≤0.5 (BKZ: 57.0%, PBO: 37.0%) (Figure), as well as for CfB in norm-based SF‑36 PCS (Table). BKZ-treated pts also showed greater improvements at Wk 16 in HRQoL (PsAQoL CfB; BKZ: –2.1, PBO: –0.2) and utility (EQ-5D-3L VAS CfB; BKZ: +11.5, PBO: +1.7) (Table). BKZ results were similar between bDMARD-naïve and TNFi-IR pts in BE OPTIMAL and BE COMPLETE, respectively (Table).
Conclusion: BKZ treatment resulted in clinically meaningful improvements in measures of HRQoL, physical function, and utility compared with PBO in pts with active PsA. Across measures, results were consistent between bDMARD-naïve and TNFi-IR pts.
References: 1. Gudu T. Expert Rev Clin Immunol 2018;14(5):405–17; 2. McInnes IB. Ann Rheum Dis 2022;81:206–7; 3. Merola JF. Ann Rheum Dis 2022;81:167–8; 4. Bakker CH. Patient Educ Couns 1993;20(2–3):145–52; 5. Krishnan E. Arthritis Rheum 2004;50(3):953–90; 6. Keystone EC. J Rheumatol 2013;40(9):1487–97.


Disclosures: D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; L. Erik, AbbVie/Abbott, Amgen, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Gilead, Janssen, Merck/MSD, Novartis, Novo Nordisk, Pfizer, UCB Pharma; D. Thaci, AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi-Genzyme, UCB Pharma, Celltrion, Morphosis, Sun Pharma; P. Gisondi, AbbVie, Abiogen, Almirall, Celgene, Eli Lilly, Janssen, LEO Pharma, Merck/MSD, Novartis, Otsuka, Pfizer, Pierre Fabre, Sanofi, UCB Pharma; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; A. Gottlieb, Amgen, AnaptsysBio, Avortres Therapeutics, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Sun Pharmaceuticals Industries, UCB Pharma, Xbiotech, Ortho; H. Dobashi, BMS, Chugai; B. Ink, UCB Pharma, GlaxoSmithKlein(GSK); D. Assudani, UCB Pharma; R. Bajracharya, UCB Pharma; J. Coarse, UCB Pharma; J. Lambert, UCB Pharma; W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB.

