Back
Poster Session D
Session: (2108–2153) Spondyloarthritis Including PsA – Treatment Poster III: PsA
2119: Bimekizumab Treatment Results in Improvements in Fatigue and Pain in Biologic DMARD-Naïve or TNFi-IR Patients with Active Psoriatic Arthritis: Pooled 16-Week Results from Two Phase 3 Randomized, Placebo-Controlled Studies
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.png)
M. Elaine Husni, MD, MPH
Cleveland Clinic
Gates Mills, OH, United States
Abstract Poster Presenter(s)
M. Elaine Husni1, Philip J Mease2, Joseph Merola3, Frank Behrens4, Ennio Giulio Favalli5, Dennis McGonagle6, William Tillett7, Shigeyoshi Tsuji8, Barbara Ink9, Deepak Assudani9, Rajan Bajracharya9, Jason Coarse10, Jérémy Lambert11 and Laure Gossec12, 1Cleveland Clinic, Cleveland, OH, 2Swedish Medical Center/Providence St. Joseph Health, Seattle, WA, 3Harvard Medical School, Brigham and Women's Hospital, Boston, MA, 4Rheumatology University Hospital & Fraunhofer Institute Translational Medicine and Pharmacology, Goethe-University Frankfurt, Frankfurt Am Main, Germany, 5University of Milan, ASST Gaetano Pini-CTO Institute, Milano, Italy, 6Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds; National Institute for Health Research, Leeds Biomedical Research Centre, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 7Royal National Hospital for Rheumatic Diseases, Bath, United Kingdom, 8Department of Orthopaedics and Rheumatology, Nippon Life Hospital, Osaka, Japan, 9UCB Pharma, Slough, United Kingdom, 10UCB Pharma, Raleigh, NC, USA, Raleigh, NC, 11UCB Pharma, Colombes, France, Irigny, France, 12Sorbonne Université, Paris, France
Background/Purpose: Patients with psoriatic arthritis (PsA) have identified fatigue and pain as important features relevant to their burden of disease.1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has demonstrated improvements in patient-reported symptoms up to 3 years in a phase 2b study.2 This analysis aimed to assess the impact of BKZ treatment vs placebo (PBO) on the clinically relevant symptoms of patient‑reported fatigue and pain in patients with active PsA who are biologic DMARD (bDMARD)-naïve or had inadequate response to 1–2 TNF inhibitors (TNFi-IR), using pooled data from BE OPTIMAL and BE COMPLETE.
Methods: BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) are phase 3 trials assessing BKZ in patients with PsA who are bDMARD-naïve or TNFi-IR, respectively. Both trials had a 16-week (wk) double-blind and placebo‑controlled phase, allowing data to be pooled across the trials. Patients in BE OPTIMAL were randomized 3:2:1 to subcutaneous (sc) BKZ 160 mg every 4 wks (Q4W), PBO, or sc adalimumab (reference arm) 40 mg every 2 weeks (Q2W); and in BE COMPLETE 2:1 to sc BKZ 160 mg Q4W or PBO. We present pooled and individual study data to Wk 16 for BKZ and PBO treatment arms. Functional Assessment of Chronic Illness Therapy-Fatigue subscale Minimum Clinically Important Difference (FACIT-Fatigue MCID; ≥4-point improvement from baseline) and clinically important improvements (≥30/50/70%) in Patient's Assessment of Arthritis Pain (PtAAP) are reported.3 Non-responder and multiple imputation (NRI, MI) were used for missing binary and continuous variables, respectively.
Results: A total of 1,073/1,112 (96.5%) patients randomized to BKZ or PBO completed Wk 16 in BE OPTIMAL and BE COMPLETE.
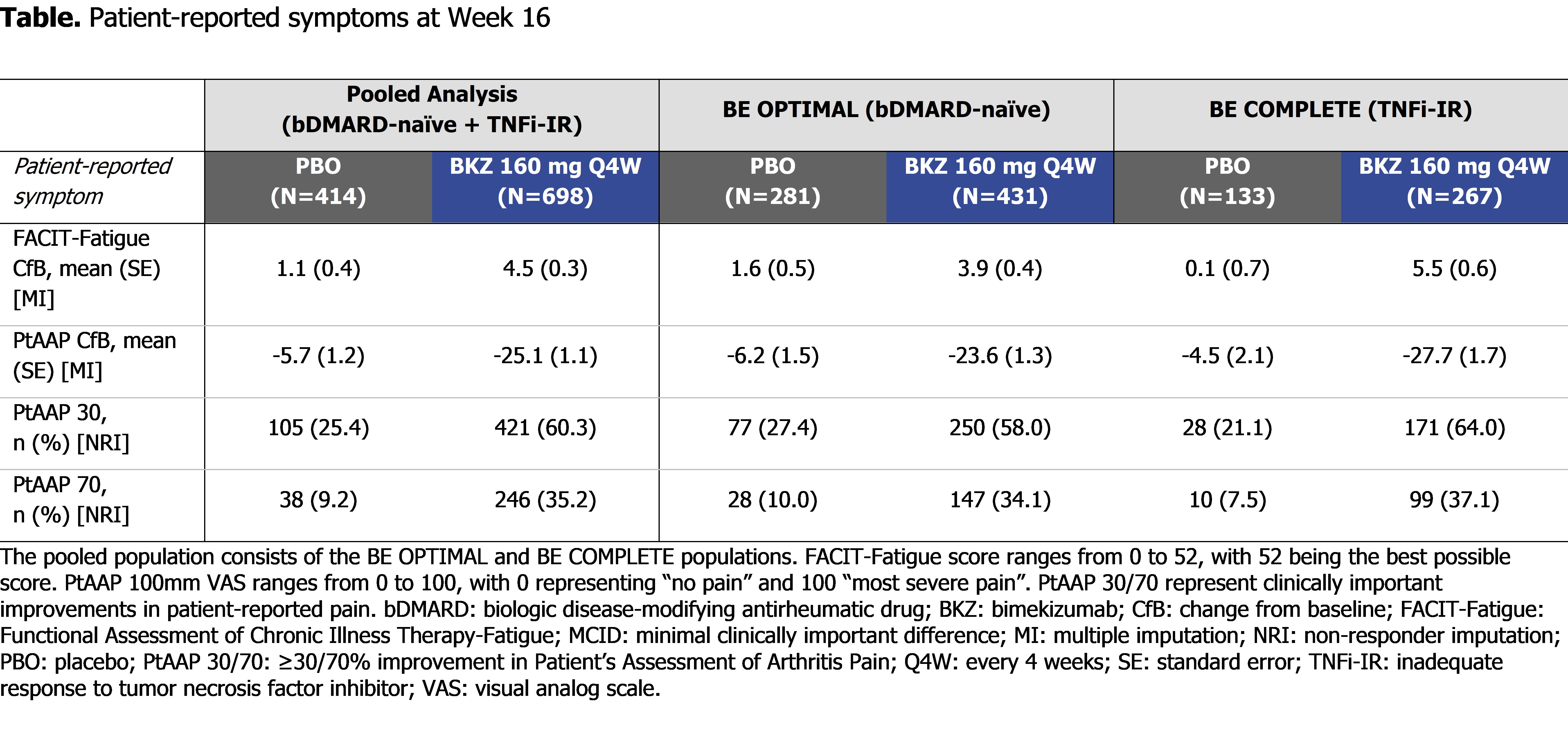
At Wk 16 (pooled), BKZ demonstrated numerically greater and clinically meaningful improvements in patient-reported fatigue and pain compared with PBO (FACIT-Fatigue MCID: 336/633 [53.1%] BKZ vs 138/380 [36.3%] PBO; PtAAP 50: 346/698 [49.6%] BKZ vs 72/414 [17.4%] PBO; Figure). BKZ-treated patients showed greater improvements in patient-reported fatigue and pain compared with PBO in the pooled population as well as the individual trial populations, meaning that results were similar between bDMARD-naïve and TNFi-IR patients (Table).
Conclusion: Bimekizumab treatment resulted in clinically meaningful improvements in patient-reported fatigue (FACIT-Fatigue) and pain (PtAAP) in approximately half of patients with active PsA who are bDMARD-naïve or TNFi-IR. Improvements were similar between bDMARD-naïve and TNFi-IR patients, suggesting that BKZ treatment leads to similar improvements in the patient-reported symptoms of fatigue and pain irrespective of prior TNFi treatment.
References: 1. Ogdie A. RMD Open 2020; 6(3):e001321; 2. Gossec L. Arthritis Rheumatol. 2021;73(suppl 10;1350); 3. Dworkin RH. J. Pain 2008; 9(2):105–21.
.jpg)
Disclosures: M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech; J. Merola, AbbVie, Biogen, BMS, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, UCB Pharma, Arena, Avotres, EMD, LEO Pharma, Merck, Regeneron, Sanofi; F. Behrens, AbbVie, Boehringer Ingelheim, Celgene, Chugai, Eli Lilly, Genzyme, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi, Bristol-Myers Squibb(BMS), Galapagos, Gilead, UCB, Affibody, MoonLake, GlaxoSmithKlein(GSK); E. Favalli, AbbVie, BMS, Celltrion, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, UCB; D. McGonagle, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, UCB, Bristol-Myers Squibb(BMS), Amgen, Gilead, Pfizer; W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; S. Tsuji, AbbVie, Eli Lilly, Janssen, Novartis, UCB; B. Ink, UCB Pharma, GlaxoSmithKlein(GSK); D. Assudani, UCB Pharma; R. Bajracharya, UCB Pharma; J. Coarse, UCB Pharma; J. Lambert, UCB Pharma; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD.
Background/Purpose: Patients with psoriatic arthritis (PsA) have identified fatigue and pain as important features relevant to their burden of disease.1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has demonstrated improvements in patient-reported symptoms up to 3 years in a phase 2b study.2 This analysis aimed to assess the impact of BKZ treatment vs placebo (PBO) on the clinically relevant symptoms of patient‑reported fatigue and pain in patients with active PsA who are biologic DMARD (bDMARD)-naïve or had inadequate response to 1–2 TNF inhibitors (TNFi-IR), using pooled data from BE OPTIMAL and BE COMPLETE.
Methods: BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) are phase 3 trials assessing BKZ in patients with PsA who are bDMARD-naïve or TNFi-IR, respectively. Both trials had a 16-week (wk) double-blind and placebo‑controlled phase, allowing data to be pooled across the trials. Patients in BE OPTIMAL were randomized 3:2:1 to subcutaneous (sc) BKZ 160 mg every 4 wks (Q4W), PBO, or sc adalimumab (reference arm) 40 mg every 2 weeks (Q2W); and in BE COMPLETE 2:1 to sc BKZ 160 mg Q4W or PBO. We present pooled and individual study data to Wk 16 for BKZ and PBO treatment arms. Functional Assessment of Chronic Illness Therapy-Fatigue subscale Minimum Clinically Important Difference (FACIT-Fatigue MCID; ≥4-point improvement from baseline) and clinically important improvements (≥30/50/70%) in Patient's Assessment of Arthritis Pain (PtAAP) are reported.3 Non-responder and multiple imputation (NRI, MI) were used for missing binary and continuous variables, respectively.
Results: A total of 1,073/1,112 (96.5%) patients randomized to BKZ or PBO completed Wk 16 in BE OPTIMAL and BE COMPLETE.
At Wk 16 (pooled), BKZ demonstrated numerically greater and clinically meaningful improvements in patient-reported fatigue and pain compared with PBO (FACIT-Fatigue MCID: 336/633 [53.1%] BKZ vs 138/380 [36.3%] PBO; PtAAP 50: 346/698 [49.6%] BKZ vs 72/414 [17.4%] PBO; Figure). BKZ-treated patients showed greater improvements in patient-reported fatigue and pain compared with PBO in the pooled population as well as the individual trial populations, meaning that results were similar between bDMARD-naïve and TNFi-IR patients (Table).
Conclusion: Bimekizumab treatment resulted in clinically meaningful improvements in patient-reported fatigue (FACIT-Fatigue) and pain (PtAAP) in approximately half of patients with active PsA who are bDMARD-naïve or TNFi-IR. Improvements were similar between bDMARD-naïve and TNFi-IR patients, suggesting that BKZ treatment leads to similar improvements in the patient-reported symptoms of fatigue and pain irrespective of prior TNFi treatment.
References: 1. Ogdie A. RMD Open 2020; 6(3):e001321; 2. Gossec L. Arthritis Rheumatol. 2021;73(suppl 10;1350); 3. Dworkin RH. J. Pain 2008; 9(2):105–21.
.jpg)

Disclosures: M. Husni, AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech; J. Merola, AbbVie, Biogen, BMS, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, UCB Pharma, Arena, Avotres, EMD, LEO Pharma, Merck, Regeneron, Sanofi; F. Behrens, AbbVie, Boehringer Ingelheim, Celgene, Chugai, Eli Lilly, Genzyme, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi, Bristol-Myers Squibb(BMS), Galapagos, Gilead, UCB, Affibody, MoonLake, GlaxoSmithKlein(GSK); E. Favalli, AbbVie, BMS, Celltrion, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, UCB; D. McGonagle, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, UCB, Bristol-Myers Squibb(BMS), Amgen, Gilead, Pfizer; W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; S. Tsuji, AbbVie, Eli Lilly, Janssen, Novartis, UCB; B. Ink, UCB Pharma, GlaxoSmithKlein(GSK); D. Assudani, UCB Pharma; R. Bajracharya, UCB Pharma; J. Coarse, UCB Pharma; J. Lambert, UCB Pharma; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD.

