Back
Poster Session D
Session: (1950–1979) RA – Diagnosis, Manifestations, and Outcomes Poster IV
1952: Burden of Disease in Refractory Rheumatoid Arthritis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Kristin Wipfler, PhD
FORWARD, The National Databank for Rheumatic Diseases
Omaha, NE, United States
Abstract Poster Presenter(s)
Kristin Wipfler1, Bobby Kwanghoon Han2, Urbano Sbarigia3, Federico Zazzetti4, Anna Sheahan5, Iris Lin6, Patricia Katz7, Evo Alemao8 and Kaleb Michaud9, 1FORWARD, The National Databank for Rheumatic Diseases, Omaha, NE, 2Division of Rheumatology, University of Washington, Seattle, WA, 3Johnson & Johnson, Beerse, Belgium, 4Janssen Medical Affairs Global Services, LLC, Buenos Aires, Argentina, 5Janssen Research and Development, Chapel Hill, NC, 6Janssen, Horsham, PA, 7UCSF, San Rafael, CA, 8Janssen, Princeton, NJ, 9University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Despite major advances in RA treatment and the improved outcomes that have been associated with the expanding number of advanced therapies available, a substantial number of patients are refractory to multiple biologics. Definitions for refractory RA (reRA) have been arbitrary and there is no universally accepted definition, but they are generally based on a specified number of failed biologics. The term "refractory" implies treatment-resistant RA with persistent inflammation, but it is often used interchangeably with broader definitions encompassing difficult to treat RA in general. Depending on the definition used and cohort studied, prevalence of reRA is estimated at 6-21%. To understand the mechanisms behind reRA and ultimately improve therapies tailored to individuals, a better understanding of outcomes and associated factors is necessary. For this reason, there is a need for more thorough analyses of the burden of refractory RA in a real-world setting.
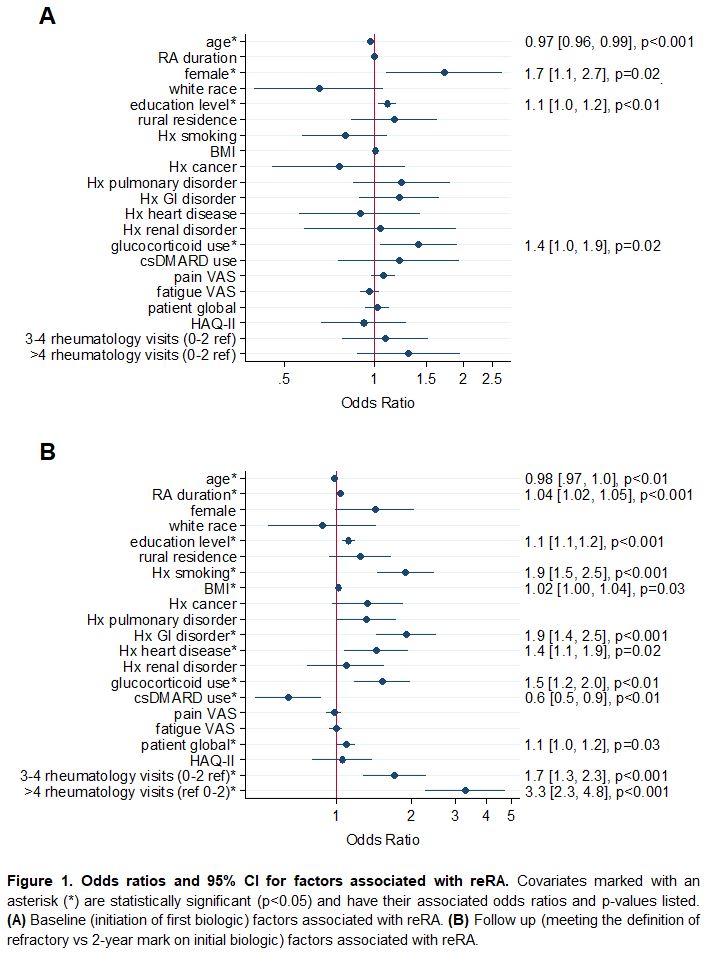
Methods: Data were provided by adults with RA in FORWARD, a longitudinal open cohort registry with comprehensive semiannual questionnaires. Participants with no history of biologic use at study entry but with subsequent exposure to one or more biologics were included. The reRA group included only participants who used at least three different biologics while under observation. Those who used a single biologic continuously for ≥2 years comprised the comparison non-refractory group. Descriptive statistics were calculated for reRA and non-refractory groups at initiation of first biologic and at the point of becoming refractory for reRA/two years after initiation of only biologic for control. Significance was assessed with Chi-square and t-tests. Characteristics at biologic initiation that were associated with becoming refractory as well as factors that varied significantly at the point of meeting reRA criteria were identified with logistic regression models adjusted for variables listed in Figure 1.
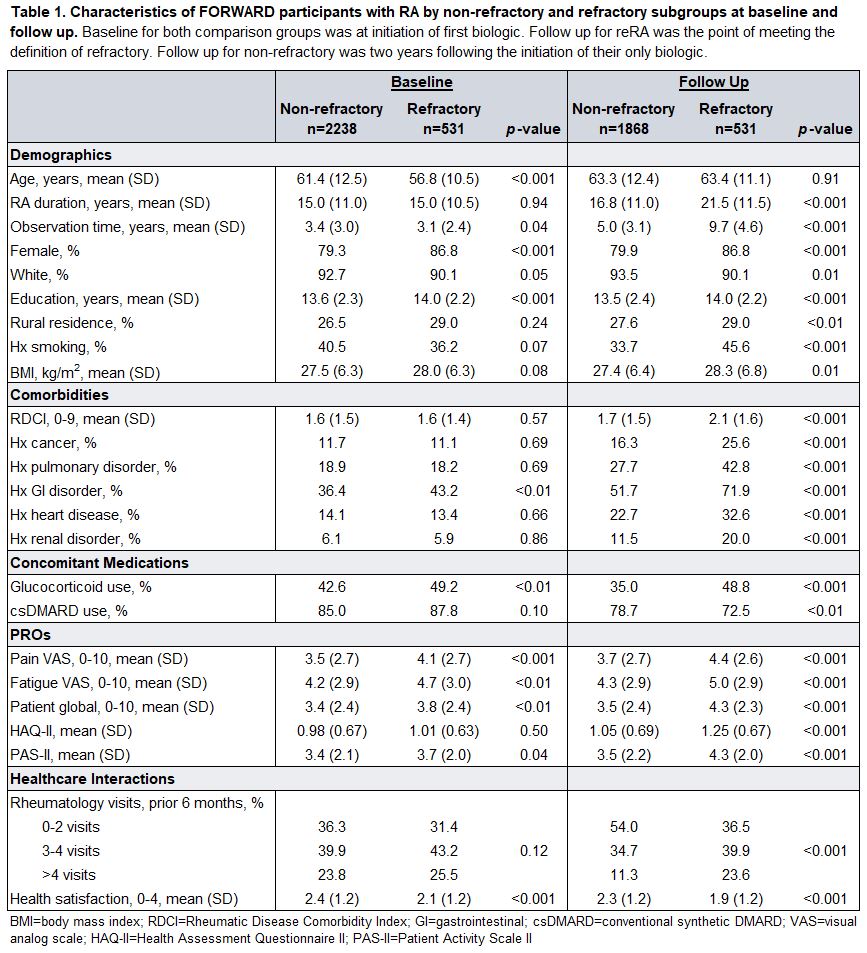
Results: Of the 2,769 participants meeting study inclusion criteria, 531 (19.2%) met criteria for reRA. Numerous baseline characteristics varied significantly by comparison group (Table 1), but some of these were attenuated in the models (Figure 1). Significant associations with future incidence of reRA included younger age, female sex, higher education, and glucocorticoid use (Figure 1A). At the time of meeting the definition of reRA, younger age, longer RA duration, higher education, history of smoking, higher BMI, GI disorder, heart disease, concomitant glucocorticoid use, lack of concomitant csDMARD use, patient global, and higher rate of rheumatology visits were associated with reRA (Figure 1B).
Conclusion: Baseline factors associated with risk of reRA were consistent with existing literature. Participants who met the definition for reRA had more comorbidities, higher rates of smoking, more frequent use of glucocorticoids, and higher health care resource use. Our findings characterize differences in disease burden and patient experience between non-refractory and reRA patients and will inform future research in refractory RA.
Disclosures: K. Wipfler, None; B. Han, None; U. Sbarigia, Janssen; F. Zazzetti, Janssen Medical Affairs Global Services, LLC; A. Sheahan, Janssen, UCB; I. Lin, Janssen, Johnson & Johnson; P. Katz, None; E. Alemao, Johnson & Johnson, Johnson & Johnson; K. Michaud, None.
Background/Purpose: Despite major advances in RA treatment and the improved outcomes that have been associated with the expanding number of advanced therapies available, a substantial number of patients are refractory to multiple biologics. Definitions for refractory RA (reRA) have been arbitrary and there is no universally accepted definition, but they are generally based on a specified number of failed biologics. The term "refractory" implies treatment-resistant RA with persistent inflammation, but it is often used interchangeably with broader definitions encompassing difficult to treat RA in general. Depending on the definition used and cohort studied, prevalence of reRA is estimated at 6-21%. To understand the mechanisms behind reRA and ultimately improve therapies tailored to individuals, a better understanding of outcomes and associated factors is necessary. For this reason, there is a need for more thorough analyses of the burden of refractory RA in a real-world setting.
Methods: Data were provided by adults with RA in FORWARD, a longitudinal open cohort registry with comprehensive semiannual questionnaires. Participants with no history of biologic use at study entry but with subsequent exposure to one or more biologics were included. The reRA group included only participants who used at least three different biologics while under observation. Those who used a single biologic continuously for ≥2 years comprised the comparison non-refractory group. Descriptive statistics were calculated for reRA and non-refractory groups at initiation of first biologic and at the point of becoming refractory for reRA/two years after initiation of only biologic for control. Significance was assessed with Chi-square and t-tests. Characteristics at biologic initiation that were associated with becoming refractory as well as factors that varied significantly at the point of meeting reRA criteria were identified with logistic regression models adjusted for variables listed in Figure 1.
Results: Of the 2,769 participants meeting study inclusion criteria, 531 (19.2%) met criteria for reRA. Numerous baseline characteristics varied significantly by comparison group (Table 1), but some of these were attenuated in the models (Figure 1). Significant associations with future incidence of reRA included younger age, female sex, higher education, and glucocorticoid use (Figure 1A). At the time of meeting the definition of reRA, younger age, longer RA duration, higher education, history of smoking, higher BMI, GI disorder, heart disease, concomitant glucocorticoid use, lack of concomitant csDMARD use, patient global, and higher rate of rheumatology visits were associated with reRA (Figure 1B).
Conclusion: Baseline factors associated with risk of reRA were consistent with existing literature. Participants who met the definition for reRA had more comorbidities, higher rates of smoking, more frequent use of glucocorticoids, and higher health care resource use. Our findings characterize differences in disease burden and patient experience between non-refractory and reRA patients and will inform future research in refractory RA.


Disclosures: K. Wipfler, None; B. Han, None; U. Sbarigia, Janssen; F. Zazzetti, Janssen Medical Affairs Global Services, LLC; A. Sheahan, Janssen, UCB; I. Lin, Janssen, Johnson & Johnson; P. Katz, None; E. Alemao, Johnson & Johnson, Johnson & Johnson; K. Michaud, None.

