Back
Ignite Talk
Session: Ignite Session 5A
1689: Oral Ginger Supplementation Counteracts NETosis in Autoimmune Mouse Models and in Healthy Humans
Sunday, November 13, 2022
1:10 PM – 1:15 PM Eastern Time
Location: Northern Liberties Stage
- RA
Ramadan Ali, PhD
University of Michigan
Ann Arobr, MI, United StatesDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Ramadan Ali1, Miela Zahavi2, Christine Rysenga1, Cyrus Sarosh3, Claire Hoy1, Kristen Demoruelle4 and Jason S Knight5, 1University of Michigan, Ann Arbor, MI, 2Universtiy of Michigan, Ann Arbor, MI, 3University of Michigan, Temperance, MI, 4University of Colorado Anschutz Medical Campus, Aurora, CO, 5University of Michigan, Division of Rheumatology, Ann Arbor, MI
Background/Purpose: It has previously been reported that 6-gingerol, the most abundant phytochemical in ginger root, inhibits neutrophil phosphodiesterase activity and thereby counteracts neutrophil hyperactivity in mouse models of antiphospholipid syndrome (APS) and lupus. In such studies, purified 6-gingerol was delivered by intraperitoneal injection. Here, we hypothesized that oral supplementation with a whole ginger extract would similarly help neutrophils resist aberrant activation.
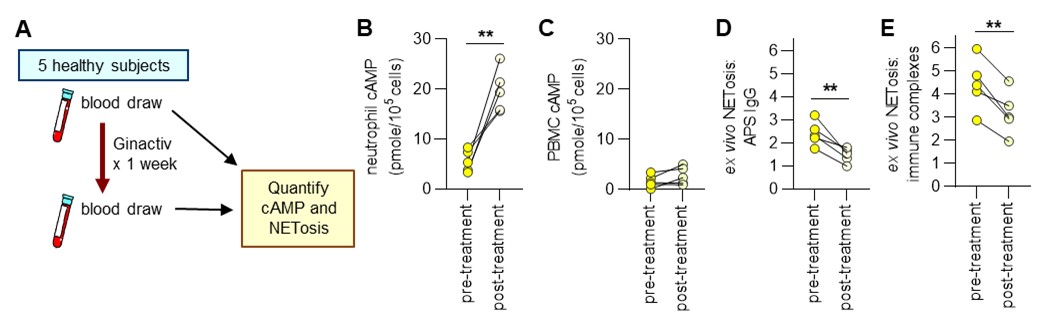
Methods: We used a commercially available ginger supplement, Ginactiv (Aurea Biolabs), which contains more gingerols than most available supplements (15-20% vs. 2-3%). To model neutrophil extracellular trap (NET)-mediated APS thrombosis, mice received human APS IgG followed by electrolytic activation of the inferior vena cava, which triggered thrombus formation over 24 hours; Ginactiv was administered by oral gavage (150 mg/kg/day). To model lupus associated NETosis, BALB/c mice were administered topical R848 (TLR7 agonist) 3 times weekly for 6 weeks; Ginactiv was incorporated into mouse chow (150 mg/kg/day). In a pilot and feasibility clinical trial (HUM00209123), 5 healthy subjects consumed Ginactiv 100 mg once daily for 7 days. Blood was sampled 1 day prior to starting the supplement and then again on day 7. At both time points, neutrophils were isolated for determination of neutrophil cAMP content and ex vivo NETosis in response to either APS IgG or lupus immune complexes.
Results: As compared with control mice, mice receiving APS IgG (n=10/group) formed larger thrombi in the inferior vena cava (control mean=4.5 mg vs. APS mean=6.6 mg; p=0.007). When ginger was administered to the APS mice, thrombus weight was markedly reduced (mean=3.8 mg; p=0.0003) as were serum NET remnants (p=0.001). In a different experiment, mice receiving R848 (n=10/group) demonstrated higher serum NET remnants (R848 mean=0.82 OD vs. control mean=0.29 OD) and anti-dsDNA antibodies (33,142 vs. 4,770 U/ml). Mice that additionally received Ginactiv had significantly lower levels of NET remnants (0.55 OD, p=0.0001) and anti-dsDNA antibodies (23,952 U/ml, p=0.009). In the pilot clinical trial (Fig 1), ginger supplementation increased mean neutrophil (but not PBMC) cAMP levels from 5.6 pmole/105 neutrophils to 19.6 pmole/105 neutrophils (p=0.0001). In parallel, ex vivo NETosis triggered by APS IgG and RNP/anti-RNP immune complexes was reduced by 45% (p=0.01) and 32% (p=0.004), respectively. There were no adverse events in any of the 5 subjects.
Conclusion: Oral administration of a ginger supplement delivers sufficient gingerols to combat APS-associated thrombosis in a NET-mediated mouse model. Ginger also reduced NETosis in a mouse model of lupus. The pilot clinical trial demonstrated that consumption of a ginger supplement by healthy individuals has the potential to alter neutrophil function in vivo resulting in neutrophils less prone to NETosis. The next step will be to study ginger supplementation in patients with APS, lupus, or other rheumatic diseases defined by neutrophil hyperactivity.
Disclosures: R. Ali, None; M. Zahavi, None; C. Rysenga, None; C. Sarosh, None; C. Hoy, None; K. Demoruelle, Boehringer-Ingelheim, Pfizer; J. Knight, Jazz Pharmaceuticals, Bristol Myers Squibb.
Background/Purpose: It has previously been reported that 6-gingerol, the most abundant phytochemical in ginger root, inhibits neutrophil phosphodiesterase activity and thereby counteracts neutrophil hyperactivity in mouse models of antiphospholipid syndrome (APS) and lupus. In such studies, purified 6-gingerol was delivered by intraperitoneal injection. Here, we hypothesized that oral supplementation with a whole ginger extract would similarly help neutrophils resist aberrant activation.
Methods: We used a commercially available ginger supplement, Ginactiv (Aurea Biolabs), which contains more gingerols than most available supplements (15-20% vs. 2-3%). To model neutrophil extracellular trap (NET)-mediated APS thrombosis, mice received human APS IgG followed by electrolytic activation of the inferior vena cava, which triggered thrombus formation over 24 hours; Ginactiv was administered by oral gavage (150 mg/kg/day). To model lupus associated NETosis, BALB/c mice were administered topical R848 (TLR7 agonist) 3 times weekly for 6 weeks; Ginactiv was incorporated into mouse chow (150 mg/kg/day). In a pilot and feasibility clinical trial (HUM00209123), 5 healthy subjects consumed Ginactiv 100 mg once daily for 7 days. Blood was sampled 1 day prior to starting the supplement and then again on day 7. At both time points, neutrophils were isolated for determination of neutrophil cAMP content and ex vivo NETosis in response to either APS IgG or lupus immune complexes.
Results: As compared with control mice, mice receiving APS IgG (n=10/group) formed larger thrombi in the inferior vena cava (control mean=4.5 mg vs. APS mean=6.6 mg; p=0.007). When ginger was administered to the APS mice, thrombus weight was markedly reduced (mean=3.8 mg; p=0.0003) as were serum NET remnants (p=0.001). In a different experiment, mice receiving R848 (n=10/group) demonstrated higher serum NET remnants (R848 mean=0.82 OD vs. control mean=0.29 OD) and anti-dsDNA antibodies (33,142 vs. 4,770 U/ml). Mice that additionally received Ginactiv had significantly lower levels of NET remnants (0.55 OD, p=0.0001) and anti-dsDNA antibodies (23,952 U/ml, p=0.009). In the pilot clinical trial (Fig 1), ginger supplementation increased mean neutrophil (but not PBMC) cAMP levels from 5.6 pmole/105 neutrophils to 19.6 pmole/105 neutrophils (p=0.0001). In parallel, ex vivo NETosis triggered by APS IgG and RNP/anti-RNP immune complexes was reduced by 45% (p=0.01) and 32% (p=0.004), respectively. There were no adverse events in any of the 5 subjects.
Conclusion: Oral administration of a ginger supplement delivers sufficient gingerols to combat APS-associated thrombosis in a NET-mediated mouse model. Ginger also reduced NETosis in a mouse model of lupus. The pilot clinical trial demonstrated that consumption of a ginger supplement by healthy individuals has the potential to alter neutrophil function in vivo resulting in neutrophils less prone to NETosis. The next step will be to study ginger supplementation in patients with APS, lupus, or other rheumatic diseases defined by neutrophil hyperactivity.

Disclosures: R. Ali, None; M. Zahavi, None; C. Rysenga, None; C. Sarosh, None; C. Hoy, None; K. Demoruelle, Boehringer-Ingelheim, Pfizer; J. Knight, Jazz Pharmaceuticals, Bristol Myers Squibb.

