Back
Poster Session D
Session: (1950–1979) RA – Diagnosis, Manifestations, and Outcomes Poster IV
1972: Comprehensive Investigation of Pain and Inflammation in Rheumatoid Arthritis from a Multidisciplinary Approach
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LG
Lilla Gunkl-Tóth, MD, PhD
University of Pécs; Semmelweis University
Budapest, Hungary
Abstract Poster Presenter(s)
Lilla Tóth1, Gergely Orsi2, Krisztina Csókási3, Gábor Sütő4, Gábor Kumánovics5, Noémi Császár-Nagy6, Szabolcs Takács7, Eszter Szigedi8, Zsófia Nagy8, Zsolt Hodovány8, Lili Duzsik8, Zoltán Vidnyánszky9, József Kun10, Péter Urbán10, György Nagy11 and Zsuzsanna Helyes12, 1Department of Pharmacology and Pharmacotherapy, Medical School, University of Pécs, Pécs, Hungary, 2MTA-PTE Clinical Neuroscience MR Research Group, Eötvös Loránd Research Network (ELKH); Department of Neurology, Medical School, University of Pecs, Pécs, Hungary, 3Institute of Psychology, Faculty of Humanities and Social Sciences, University of Pécs, Pécs, Hungary, 4Second Department of Medicine and Nephrology-Diabetes Centre, University of Pécs, Pécs, Hungary, 5Department of Rheumatology and Immunology, University of Pécs, Pécs, Hungary, 6National University of Public Services; Psychosomatic Outpatient Clinics, Budapest, Hungary, 7Department of Psychology, Karoly Gaspar University, Budapest, Hungary, 8Psychosomatic Outpatient Clinics, Budapest, Hungary, 9Brain Imaging Centre, Research Centre for Natural Sciences, Budapest, Hungary, 10Szentágothai Research Centre, Bioinformatics Research Group, Genomics and Bioinformatics Core Facility, University of Pécs, Pécs, Hungary, 11Department of Rheumatology and Clinical Immunology, Department of Internal Medicine and Oncology, Semmelweis University; Department of Genetics, Cell and Immunobiology, Semmelweis University; Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, 12Department of Pharmacology and Pharmacotherapy, Medical School, University of Pécs; Szentágothai Research Centre and Centre for Neuroscience, University of Pécs, Pécs, Hungary
Background/Purpose: Despite the therapeutic advances of rheumatoid arthritis (RA), optimal disease control cannot be achieved in the difficult-to-treat (D2T) RA population, leading to the persistence of symptoms, especially pain. Multiple factors contribute to pain in RA, including inflammation, dysfunctions of the ascending pain processing and descending inhibitory pathways, structural damage, as well as physical and psycho-social comorbidities. However, the pathophysiological mechanisms are still unclear. Our aim is to examine the processes in the background of RA pain with a multidisciplinary approach and to reveal the interactions among pain, inflammation, psychological and social factors.
Methods: 11 healthy controls (HC) and 14 RA patients fulfilling the 2020 EULAR definition to D2T RA were included. Clinical examination including musculoskeletal ultrasound, psychological analysis (personal clinical interview, Rorschach test and validated surveys) and functional MRI (fMRI) with standardized painful heat stimulation was performed. Blood was drawn for diagnostic and experimental purposes. (Figure1)
Results: The clinical examinations identified 3 subpopulations within D2T RA on the basis of the intensities of the inflammatory reaction and pain (Table1). Our psychological survey showed that RA patients had higher rate of depression, experienced somatic complaints, had trouble in movement, movement phobia, an increased proportion of pain, avoidance of painful activities, sensitivity to pain, and dependence. Among the pathological cognitive schemes: "vulnerability", "feeling of fragmentation (isolation)", "phantasy of rejection" scheme and decreased "autonomy and work ability" scheme was detected compared to HC. Patients have higher trait anxiety; however, not free-floating anxiety (GAD), but anxiety about movement and performance. In general, their psychological feelings are also decreased. This untreated pain focused cognitive nonadaptive negative fixations, anxiety dissociated in somatic perceptions and mood disorders could lead to the failure of medical therapies. fMRI examinations revealed several differences in the activation pattern of pain related structures between HC and RA populations. After painful stimulation, the functional connectivity strength (FCS) between prefrontal and posterior cingulate cortices was reduced in HC, whereas it increased in RA patients. FCS within the default mode network (DMN) and between DMN and frontoparietal network, as well as in several connections of the anterior medial prefrontal cortex (aMPFC) were also reduced in HC. This pattern was not present in patients, moreover, pain rather increased the FCS between aMPFC and lateral occipital cortex in the RA population. PBMC isolation and RNA extraction reached high quality in all samples, transcriptomic analysis is currently going on to determine pain-related pathways, mediators and targets.
Conclusion: We revealed several abnormalities in the pain processing pathway and pain matrix activation in the brain of RA patients with persistent pain compared to HC. Psycho-social factors also strongly influence the perception of pain in RA.
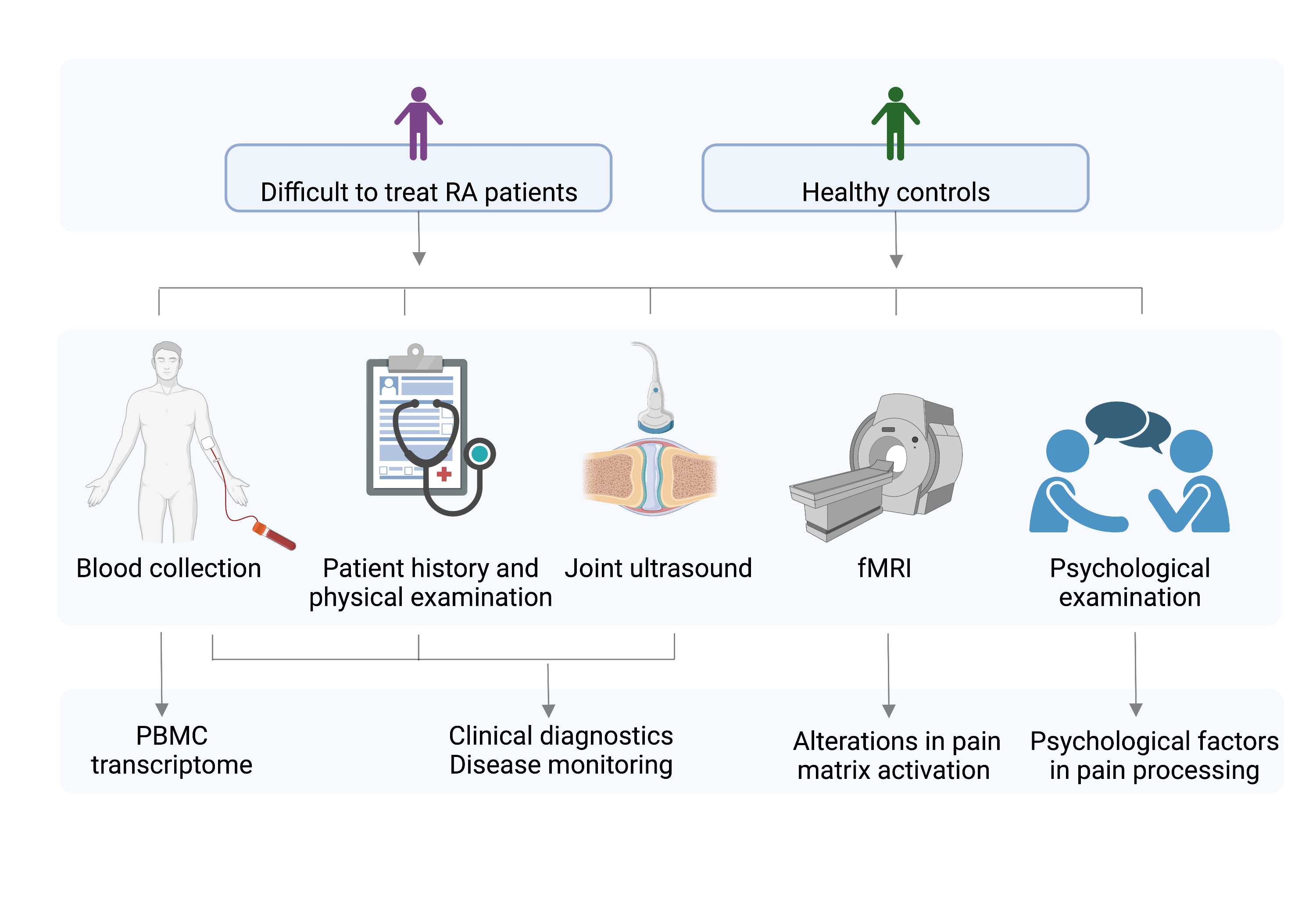 Figure 1. Study design;
Figure 1. Study design;
Difficult-to-treat RA patients: according to EULAR definition of diffictult-to-treat RA
PBMC: peripheral blood mononuclear cells, fMRI: functional MRI
Created with biorender.com
.jpg) Table 1. Clinical and demographical summary;
Table 1. Clinical and demographical summary;
Low inflammatory activity, high pain: DAS28 ≥ 3,2 AND CRP ≤ 5 mg/L OR ESR ≤20 mm/h AND VAS ≥ 60 mm; High inflammatory activity, low pain: DAS28 ≥ 3,2 AND CRP > 5 mg/L OR ESR > 20 mm/h AND VAS ≤ 60 mm; High inflammatory activity, high pain: DAS28 ≥ 3,2 AND CRP > 5 mg/L OR ESR > 20 mm/h AND VAS ≥ 60 mm
RA: rheumatoid arhtritis; SEM: standard error of the mean; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; TJC: tender joint count SJC: swollen joint count; VAS: visual analogue scale (0-100 mm), PtGA: Patient's Global Assessment of Disease Activity (higher scores indicate increased arthritis disease activity) PGA: Physician's Global Assessment of Disease Activity (higher scores indicate increased arthritis disease activity); PtGH: Patient's Assessment of Global Health (higher scores indicate decreased global health); DAS28-CRP: disease activity score 28 using C-reactive protein; DAS28-ESR: disease activity score 28 using erythrocyte sedimentation rate; SDAI: simple disease activity index; CDAI: clinical disease activity index; Comorbid fibromyalgia is based on the 2016 revision of the American College of Rheumatology 2010/2011 diagnostic criteria (Widespread pain index (WPI) ≥ 7 and symptom severity scale (SSS) score ≥ 5 OR WPI of 4–6 and SSS score ≥9 AND widespread pain affecting at least 4/5 body regions AND symptoms have been present for at least 3 months); Neuropathic pain possibility and likeliness is based on the PainDETECT questionnaire
Disclosures: L. Tóth, None; G. Orsi, None; K. Csókási, None; G. Sütő, None; G. Kumánovics, None; N. Császár-Nagy, None; S. Takács, None; E. Szigedi, None; Z. Nagy, None; Z. Hodovány, None; L. Duzsik, None; Z. Vidnyánszky, None; J. Kun, None; P. Urbán, None; G. Nagy, None; Z. Helyes, None.
Background/Purpose: Despite the therapeutic advances of rheumatoid arthritis (RA), optimal disease control cannot be achieved in the difficult-to-treat (D2T) RA population, leading to the persistence of symptoms, especially pain. Multiple factors contribute to pain in RA, including inflammation, dysfunctions of the ascending pain processing and descending inhibitory pathways, structural damage, as well as physical and psycho-social comorbidities. However, the pathophysiological mechanisms are still unclear. Our aim is to examine the processes in the background of RA pain with a multidisciplinary approach and to reveal the interactions among pain, inflammation, psychological and social factors.
Methods: 11 healthy controls (HC) and 14 RA patients fulfilling the 2020 EULAR definition to D2T RA were included. Clinical examination including musculoskeletal ultrasound, psychological analysis (personal clinical interview, Rorschach test and validated surveys) and functional MRI (fMRI) with standardized painful heat stimulation was performed. Blood was drawn for diagnostic and experimental purposes. (Figure1)
Results: The clinical examinations identified 3 subpopulations within D2T RA on the basis of the intensities of the inflammatory reaction and pain (Table1). Our psychological survey showed that RA patients had higher rate of depression, experienced somatic complaints, had trouble in movement, movement phobia, an increased proportion of pain, avoidance of painful activities, sensitivity to pain, and dependence. Among the pathological cognitive schemes: "vulnerability", "feeling of fragmentation (isolation)", "phantasy of rejection" scheme and decreased "autonomy and work ability" scheme was detected compared to HC. Patients have higher trait anxiety; however, not free-floating anxiety (GAD), but anxiety about movement and performance. In general, their psychological feelings are also decreased. This untreated pain focused cognitive nonadaptive negative fixations, anxiety dissociated in somatic perceptions and mood disorders could lead to the failure of medical therapies. fMRI examinations revealed several differences in the activation pattern of pain related structures between HC and RA populations. After painful stimulation, the functional connectivity strength (FCS) between prefrontal and posterior cingulate cortices was reduced in HC, whereas it increased in RA patients. FCS within the default mode network (DMN) and between DMN and frontoparietal network, as well as in several connections of the anterior medial prefrontal cortex (aMPFC) were also reduced in HC. This pattern was not present in patients, moreover, pain rather increased the FCS between aMPFC and lateral occipital cortex in the RA population. PBMC isolation and RNA extraction reached high quality in all samples, transcriptomic analysis is currently going on to determine pain-related pathways, mediators and targets.
Conclusion: We revealed several abnormalities in the pain processing pathway and pain matrix activation in the brain of RA patients with persistent pain compared to HC. Psycho-social factors also strongly influence the perception of pain in RA.
 Figure 1. Study design;
Figure 1. Study design;Difficult-to-treat RA patients: according to EULAR definition of diffictult-to-treat RA
PBMC: peripheral blood mononuclear cells, fMRI: functional MRI
Created with biorender.com
.jpg) Table 1. Clinical and demographical summary;
Table 1. Clinical and demographical summary;Low inflammatory activity, high pain: DAS28 ≥ 3,2 AND CRP ≤ 5 mg/L OR ESR ≤20 mm/h AND VAS ≥ 60 mm; High inflammatory activity, low pain: DAS28 ≥ 3,2 AND CRP > 5 mg/L OR ESR > 20 mm/h AND VAS ≤ 60 mm; High inflammatory activity, high pain: DAS28 ≥ 3,2 AND CRP > 5 mg/L OR ESR > 20 mm/h AND VAS ≥ 60 mm
RA: rheumatoid arhtritis; SEM: standard error of the mean; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; TJC: tender joint count SJC: swollen joint count; VAS: visual analogue scale (0-100 mm), PtGA: Patient's Global Assessment of Disease Activity (higher scores indicate increased arthritis disease activity) PGA: Physician's Global Assessment of Disease Activity (higher scores indicate increased arthritis disease activity); PtGH: Patient's Assessment of Global Health (higher scores indicate decreased global health); DAS28-CRP: disease activity score 28 using C-reactive protein; DAS28-ESR: disease activity score 28 using erythrocyte sedimentation rate; SDAI: simple disease activity index; CDAI: clinical disease activity index; Comorbid fibromyalgia is based on the 2016 revision of the American College of Rheumatology 2010/2011 diagnostic criteria (Widespread pain index (WPI) ≥ 7 and symptom severity scale (SSS) score ≥ 5 OR WPI of 4–6 and SSS score ≥9 AND widespread pain affecting at least 4/5 body regions AND symptoms have been present for at least 3 months); Neuropathic pain possibility and likeliness is based on the PainDETECT questionnaire
Disclosures: L. Tóth, None; G. Orsi, None; K. Csókási, None; G. Sütő, None; G. Kumánovics, None; N. Császár-Nagy, None; S. Takács, None; E. Szigedi, None; Z. Nagy, None; Z. Hodovány, None; L. Duzsik, None; Z. Vidnyánszky, None; J. Kun, None; P. Urbán, None; G. Nagy, None; Z. Helyes, None.

