Back
Poster Session D
Session: (2108–2153) Spondyloarthritis Including PsA – Treatment Poster III: PsA
2128: Consistent Long-Term Guselkumab Efficacy Across Psoriatic Arthritis Domains Irrespective of Baseline Patient Characteristics
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- IM
Iain McInnes, PhD, FRCP
Professor of Medicine and Rheumatology
University of Glasgow
Glasgow, United Kingdom
Abstract Poster Presenter(s)
Iain B McInnes1, John Tesser2, Elena Schiopu3, Joseph Merola4, Soumya Chakravarty5, Emmanouil Rampakakis6, Natalie Shiff7, Alexa Kollmeier8, Xie L Xu9, May Shawi10, Frederic Lavie11, Paul Bird12 and Philip J Mease13, 1Institute of Infection, Immunity and Inflammation, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, Scotland, United Kingdom, 2Arizona Arthritis & Rheumatology Associates, Phoenix, AZ, 3Michigan Medicine Rheumatology Clinic – Taubman Center, Ann Arbor, MI, 4Brigham and Women's Hospital, Harvard Medical School, Boston, MA, 5Janssen Scientific Affairs, LLC; Drexel University College of Medicine, Villanova, PA, 6McGill University, Department of Pediatrics and JSS Medical Research, Montréal, QC, Canada, 7Janssen Scientific Affairs, LLC; Community Health and Epidemiology, University of Saskatchewan, Philadelphia, PA, 8Janssen-Cilag, Research & Development, LLC, San Diego, CA, 9Janssen Research & Development, LLC, San Diego, CA, USA, San Marcos, CA, 10Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, Horsham, PA, 11The Janssen Pharmaceutical Companies of Johnson & Johnson, Paris, France, Paris, France, 12University of New South Wales, Sydney, Australia, 13Swedish Medical Center/Providence St. Joseph Health, Seattle, WA
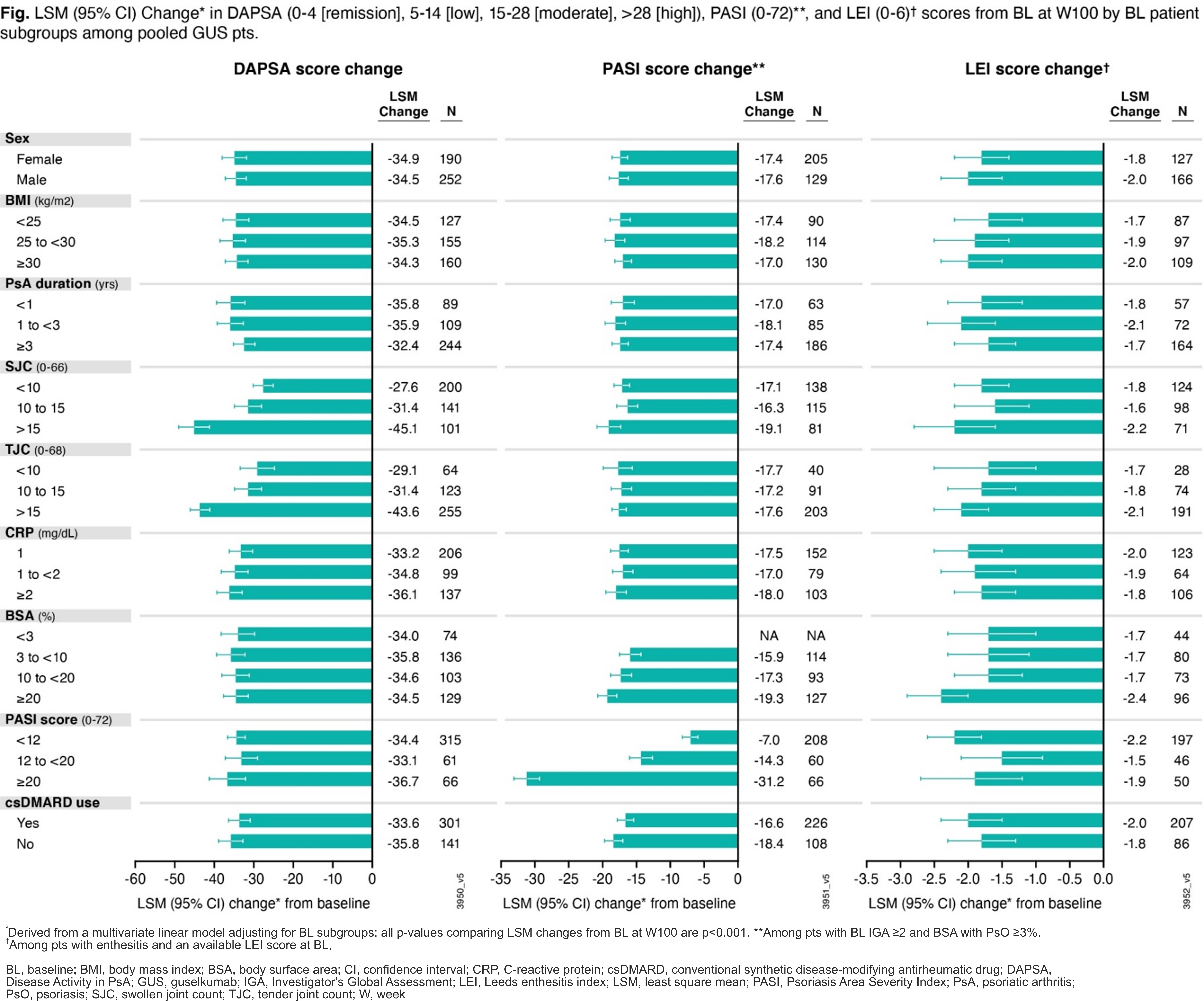
Background/Purpose: In the phase 3 DISCOVER (D)-1 and D-2 studies, guselkumab (GUS) significantly improved joint symptoms, skin disease, enthesitis, dactylitis, physical function, and quality of life at Week (W)24 in patients (pts) with psoriatic arthritis (PsA).1,2 Clinical responses across these disease domains were maintained or increased with GUS at W52,3,4 regardless of baseline (BL) pt demographics, disease characteristics, or conventional synthetic disease-modifying antirheumatic drug (csDMARD) use.5 Durable efficacy with GUS through W100 across multiple disease domains was observed.6 This study assessed both BL predictors of, and by BL pt subgroups, GUS efficacy across PsA disease domains through W100 of D-2.
Methods: Biologic-naïve pts with active PsA despite standard therapies in D-2 (swollen joint count [SJC] ≥5 & tender joint count [TJC] ≥5, C-reactive protein [CRP] ≥0.6 mg/dL) were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo (PBO).2 GUS effects on joint, skin, enthesitis, dactylitis, spinal pain, and disease severity endpoints (change in Disease Activity in PsA [DAPSA], SJC, and TJC scores; Psoriasis [PsO] Area Severity Index [PASI] score [among pts with BL IGA ≥2 and body surface area [BSA] with PsO ≥3%]; Leeds enthesitis index [LEI] score; dactylitis score; spinal pain score; and PsA Disease Activity Score [PASDAS], respectively) at W100 were evaluated for GUS-randomized pts. A multivariate linear model adjusting for BL pt characteristics assessed associations between BL predictors and changes in DAPSA, PASI, and LEI scores from BL to W100, and least squares mean changes and 95% confidence intervals in all continuous endpoints from BL to W100 within subgroups of pts defined by BL sex, body mass index, PsA duration, SJC, TJC, CRP, %BSA, PASI score, and csDMARD use.
Results: 442 (90%) GUS-randomized pts completed study treatment through W100.6 Among the BL predictors of long-term GUS efficacy assessed, only PsA duration (p=0.032), SJC (p< 0.001), and TJC (p< 0.001) were significant predictors of long-term (BL to W100) DAPSA score change; %BSA (p=0.002), PASI score (p< 0.001), SJC (p=0.008), and csDMARD use (p=0.014) were significant predictors of long-term PASI score change; and none significantly predicted long-term LEI score change among pooled GUS pts (Fig). However, statistically significant improvements from BL to W100 in DAPSA, PASI, and LEI scores were observed across all BL strata in pooled GUS Q4W+Q8W pts (Fig, all p< 0.001) and within each dosing group. Similar improvements were observed for other continuous endpoints assessed (change in PASDAS, SJC, TJC, spinal pain, and dactylitis score).
Conclusion: GUS significantly improved PsA signs and symptoms through W100 across all BL pt subgroups including pts with highly active disease regardless of dosing regimen.
References:
1. Deodhar A et al. Lancet 2020; 395:1115-25
2. Mease PJ et al. Lancet 2020; 395;1126-36
3. Ritchlin CT et al. RMD Open 2021;7(1):e001457
4. McInnes IB et al. Arthritis Rheumatol 2021; 73:604-16
5. Ritchlin CT et al. Ann Rheum Dis 2021; 80:1291-2
6. McInnes IB et al. Arthritis Rheumatol 2021; doi: 10.1002/art.42010
Disclosures: I. McInnes, Bristol-Myers Squibb (BMS), Janssen, Novartis, UCB, Pfizer, AbbVie, Celgene, AstraZeneca, Boehringer Ingelheim, EveloBio, LEO, Lilly; J. Tesser, AbbVie, Amgen, Bristol-Myers Squibb(BMS), Celgene, CoreVitas, Eli Lilly, Gilead, Janssen, Pfizer, Sun Pharma, Novartis; E. Schiopu, Janssen; J. Merola, Novartis, Pfizer, Sun Pharma, UCB Pharma, Bristol-Myers Squibb(BMS), AbbVie, Dermavant, Eli Lilly, Janssen, Arena, Biogen; S. Chakravarty, Janssen Scientific Affairs, LLC, Johnson & Johnson; E. Rampakakis, Janssen, JSS Medical Research; N. Shiff, AbbVie/Abbott, Gilead, Johnson & Johnson, Janssen Scientific Affairs, LLC; A. Kollmeier, Janssen Research & Development, LLC, Johnson & Johnson; X. Xu, Janssen Research & Development, LLC, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; F. Lavie, Janssen; P. Bird, AbbVie/Abbott, Eli Lilly, Gilead, Janssen, Merck/MSD, Pfizer, UCB, Novartis; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.
Background/Purpose: In the phase 3 DISCOVER (D)-1 and D-2 studies, guselkumab (GUS) significantly improved joint symptoms, skin disease, enthesitis, dactylitis, physical function, and quality of life at Week (W)24 in patients (pts) with psoriatic arthritis (PsA).1,2 Clinical responses across these disease domains were maintained or increased with GUS at W52,3,4 regardless of baseline (BL) pt demographics, disease characteristics, or conventional synthetic disease-modifying antirheumatic drug (csDMARD) use.5 Durable efficacy with GUS through W100 across multiple disease domains was observed.6 This study assessed both BL predictors of, and by BL pt subgroups, GUS efficacy across PsA disease domains through W100 of D-2.
Methods: Biologic-naïve pts with active PsA despite standard therapies in D-2 (swollen joint count [SJC] ≥5 & tender joint count [TJC] ≥5, C-reactive protein [CRP] ≥0.6 mg/dL) were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo (PBO).2 GUS effects on joint, skin, enthesitis, dactylitis, spinal pain, and disease severity endpoints (change in Disease Activity in PsA [DAPSA], SJC, and TJC scores; Psoriasis [PsO] Area Severity Index [PASI] score [among pts with BL IGA ≥2 and body surface area [BSA] with PsO ≥3%]; Leeds enthesitis index [LEI] score; dactylitis score; spinal pain score; and PsA Disease Activity Score [PASDAS], respectively) at W100 were evaluated for GUS-randomized pts. A multivariate linear model adjusting for BL pt characteristics assessed associations between BL predictors and changes in DAPSA, PASI, and LEI scores from BL to W100, and least squares mean changes and 95% confidence intervals in all continuous endpoints from BL to W100 within subgroups of pts defined by BL sex, body mass index, PsA duration, SJC, TJC, CRP, %BSA, PASI score, and csDMARD use.
Results: 442 (90%) GUS-randomized pts completed study treatment through W100.6 Among the BL predictors of long-term GUS efficacy assessed, only PsA duration (p=0.032), SJC (p< 0.001), and TJC (p< 0.001) were significant predictors of long-term (BL to W100) DAPSA score change; %BSA (p=0.002), PASI score (p< 0.001), SJC (p=0.008), and csDMARD use (p=0.014) were significant predictors of long-term PASI score change; and none significantly predicted long-term LEI score change among pooled GUS pts (Fig). However, statistically significant improvements from BL to W100 in DAPSA, PASI, and LEI scores were observed across all BL strata in pooled GUS Q4W+Q8W pts (Fig, all p< 0.001) and within each dosing group. Similar improvements were observed for other continuous endpoints assessed (change in PASDAS, SJC, TJC, spinal pain, and dactylitis score).
Conclusion: GUS significantly improved PsA signs and symptoms through W100 across all BL pt subgroups including pts with highly active disease regardless of dosing regimen.
References:
1. Deodhar A et al. Lancet 2020; 395:1115-25
2. Mease PJ et al. Lancet 2020; 395;1126-36
3. Ritchlin CT et al. RMD Open 2021;7(1):e001457
4. McInnes IB et al. Arthritis Rheumatol 2021; 73:604-16
5. Ritchlin CT et al. Ann Rheum Dis 2021; 80:1291-2
6. McInnes IB et al. Arthritis Rheumatol 2021; doi: 10.1002/art.42010

Disclosures: I. McInnes, Bristol-Myers Squibb (BMS), Janssen, Novartis, UCB, Pfizer, AbbVie, Celgene, AstraZeneca, Boehringer Ingelheim, EveloBio, LEO, Lilly; J. Tesser, AbbVie, Amgen, Bristol-Myers Squibb(BMS), Celgene, CoreVitas, Eli Lilly, Gilead, Janssen, Pfizer, Sun Pharma, Novartis; E. Schiopu, Janssen; J. Merola, Novartis, Pfizer, Sun Pharma, UCB Pharma, Bristol-Myers Squibb(BMS), AbbVie, Dermavant, Eli Lilly, Janssen, Arena, Biogen; S. Chakravarty, Janssen Scientific Affairs, LLC, Johnson & Johnson; E. Rampakakis, Janssen, JSS Medical Research; N. Shiff, AbbVie/Abbott, Gilead, Johnson & Johnson, Janssen Scientific Affairs, LLC; A. Kollmeier, Janssen Research & Development, LLC, Johnson & Johnson; X. Xu, Janssen Research & Development, LLC, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; F. Lavie, Janssen; P. Bird, AbbVie/Abbott, Eli Lilly, Gilead, Janssen, Merck/MSD, Pfizer, UCB, Novartis; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.

