Back
Ignite Talk
Session: Ignite Session 1C
1162: Ignite Session 5C
Saturday, November 12, 2022
1:10 PM – 1:15 PM Eastern Time
Location: South Philly Stage
.png)
Denis Poddubnyy, MD
Charité-Universitätsmedizin Berlin
Berlin, GermanyDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
valeria Rios-Rodriguez1, Morgan Essex2, Judith Rademacher3, Murat Torgutalp4, Fabian Proft5, Ulrike Löber2, Lajos Marko2, Sofia Forslund2 and Denis Poddubnyy5, 1Charité-Universitätsmedizin Berlin, Berlin, Germany, 2Max Delbrück Center for Molecular Medicine (MDC) in the Helmholtz Association, Berlin, Germany, 3Charité Universitätsmedizin Berlin, Berlin, Germany, 4Charité - Universitätsmedizin, Berlin, Berlin, Germany, 5Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany
Background/Purpose: There is little evidence about the effect of biological disease-modifying antirheumatic drugs (bDMARD) on gut dysbiosis in patients with axial SpA. Here we investigated the gut microbiota changes in patients with axial SpA after receiving one year of treatment with bDMARDs.
Methods: Patients with radiographic axial SpA were recruited between 2015 and 2019 in an extension of the prospective German Spondyloarthritis Inception Cohort (GESPIC) before beginning bDMARD therapy. All patients had high disease activity (BASDAI >=4 and/or ASDAS >=2.1) despite previous treatment with nonsteroidal anti-inflammatory drugs, and had not received treatment with bDMARDs for at least three months before enrollment in the study. The choice of bDMARD was left to the discretion of the clinical rheumatologists in accordance with standard practice. Disease activity measures (BASDAI, CRP and ASDAS) and fecal samples were assessed at baseline prior to treatment and after one year of treatment. Patients with back pain negative for inflammatory disease served as a control group. Microbiota composition was determined by 16S rRNA gene sequencing, followed by taxonomic profiling with the SILVA138 database.
Results: A total of 99 patients with axial SpA and 63 control individuals were included based on the availability of clinical and microbiome samples. As general characteristics, average age was 36.4±10.4 years, 89.9% of SpA patients were HLA-B27 positive (compared to 7.9% of controls) and 64 patients were males. 97.9% of patients were treated with TNF inhibitors and 2.1% with anti-interleukin-17 agents.
At the genus level, patients were mainly depleted in Lachnospiraceae taxa such as Blautia, Roseburia, and Fusicatenibacter, and enriched in Collinsella compared to the control group at baseline. After one year of treatment, most SpA patients exhibited increased abundances of these taxa, most notably Blautia. Collinsella showed also a very slight median increase after one year of treatment, independently of disease activity. Patients also exhibited depletions in Bacteroides and Faecalibacterium, which was strongly enriched in HLA-B27+ individuals at baseline (adjusted Wilcoxon p< 0.001). Shifts in highly abundant Prevotella and Bacteroides were strongly correlated with the change in ASDAS after one year when controlling for intra-individual variance and overall changes in alpha diversity.
Simpson indices showed an increase in alpha diversity between baseline and year 1 in SpA patients which was statistically insignificant (paired Wilcoxon p=0.154) but brought the SpA cohort nearer to controls. Likewise, Bray-Curtis dissimilarities to measure beta diversity showed a qualitative normalization to healthy individuals after treatment when visualized in principal coordinate space.
Conclusion: The gut microbiota composition of axial SpA patients who underwent treatment with bDMARDs for one year more closely resembled controls. The unique enrichment of Collinsella in axial SpA patients remained stable across time and treatment, suggesting it may be a disease biomarker.
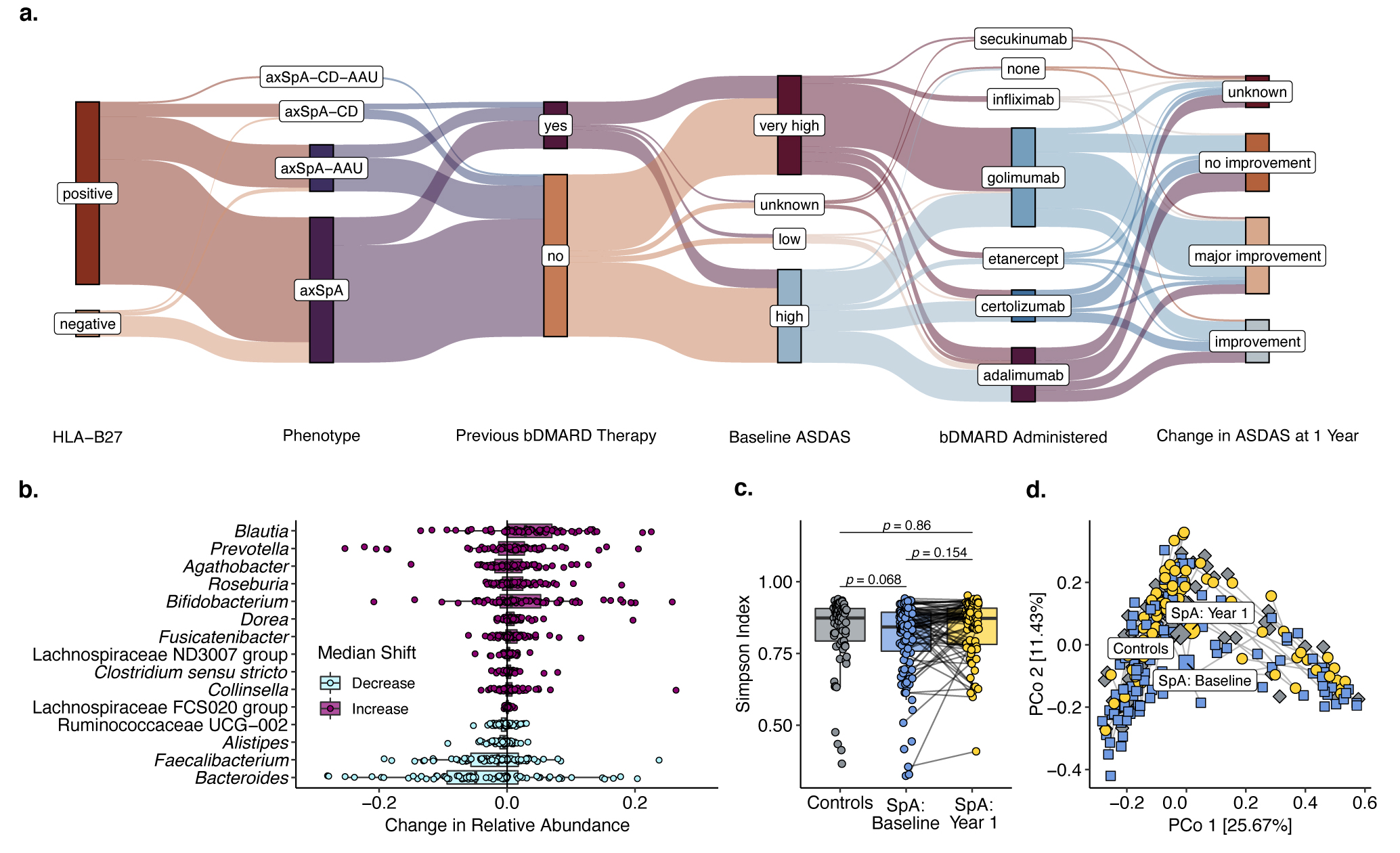 Figure. a) Flowchart of axial SpA patients summarizing the main clinical and disease activity parameters of the cohort. b) Taxa with the most pronounced shifts in median relative abundance in patients with axial SpA after receiving biological treatment for one year. c-d) Alpha and beta diversity analyses, respectively, of axial SpA patients before and after treatment compared to control individuals. Labeled points in d represent group means.
Figure. a) Flowchart of axial SpA patients summarizing the main clinical and disease activity parameters of the cohort. b) Taxa with the most pronounced shifts in median relative abundance in patients with axial SpA after receiving biological treatment for one year. c-d) Alpha and beta diversity analyses, respectively, of axial SpA patients before and after treatment compared to control individuals. Labeled points in d represent group means.
Disclosures: v. Rios-Rodriguez, Falk, AbbVie/Abbott; M. Essex, None; J. Rademacher, Novartis, UCB; M. Torgutalp, UCB, AbbVie/Abbott; F. Proft, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, Roche, UCB; U. Löber, None; L. Marko, None; S. Forslund, None; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.
Background/Purpose: There is little evidence about the effect of biological disease-modifying antirheumatic drugs (bDMARD) on gut dysbiosis in patients with axial SpA. Here we investigated the gut microbiota changes in patients with axial SpA after receiving one year of treatment with bDMARDs.
Methods: Patients with radiographic axial SpA were recruited between 2015 and 2019 in an extension of the prospective German Spondyloarthritis Inception Cohort (GESPIC) before beginning bDMARD therapy. All patients had high disease activity (BASDAI >=4 and/or ASDAS >=2.1) despite previous treatment with nonsteroidal anti-inflammatory drugs, and had not received treatment with bDMARDs for at least three months before enrollment in the study. The choice of bDMARD was left to the discretion of the clinical rheumatologists in accordance with standard practice. Disease activity measures (BASDAI, CRP and ASDAS) and fecal samples were assessed at baseline prior to treatment and after one year of treatment. Patients with back pain negative for inflammatory disease served as a control group. Microbiota composition was determined by 16S rRNA gene sequencing, followed by taxonomic profiling with the SILVA138 database.
Results: A total of 99 patients with axial SpA and 63 control individuals were included based on the availability of clinical and microbiome samples. As general characteristics, average age was 36.4±10.4 years, 89.9% of SpA patients were HLA-B27 positive (compared to 7.9% of controls) and 64 patients were males. 97.9% of patients were treated with TNF inhibitors and 2.1% with anti-interleukin-17 agents.
At the genus level, patients were mainly depleted in Lachnospiraceae taxa such as Blautia, Roseburia, and Fusicatenibacter, and enriched in Collinsella compared to the control group at baseline. After one year of treatment, most SpA patients exhibited increased abundances of these taxa, most notably Blautia. Collinsella showed also a very slight median increase after one year of treatment, independently of disease activity. Patients also exhibited depletions in Bacteroides and Faecalibacterium, which was strongly enriched in HLA-B27+ individuals at baseline (adjusted Wilcoxon p< 0.001). Shifts in highly abundant Prevotella and Bacteroides were strongly correlated with the change in ASDAS after one year when controlling for intra-individual variance and overall changes in alpha diversity.
Simpson indices showed an increase in alpha diversity between baseline and year 1 in SpA patients which was statistically insignificant (paired Wilcoxon p=0.154) but brought the SpA cohort nearer to controls. Likewise, Bray-Curtis dissimilarities to measure beta diversity showed a qualitative normalization to healthy individuals after treatment when visualized in principal coordinate space.
Conclusion: The gut microbiota composition of axial SpA patients who underwent treatment with bDMARDs for one year more closely resembled controls. The unique enrichment of Collinsella in axial SpA patients remained stable across time and treatment, suggesting it may be a disease biomarker.
 Figure. a) Flowchart of axial SpA patients summarizing the main clinical and disease activity parameters of the cohort. b) Taxa with the most pronounced shifts in median relative abundance in patients with axial SpA after receiving biological treatment for one year. c-d) Alpha and beta diversity analyses, respectively, of axial SpA patients before and after treatment compared to control individuals. Labeled points in d represent group means.
Figure. a) Flowchart of axial SpA patients summarizing the main clinical and disease activity parameters of the cohort. b) Taxa with the most pronounced shifts in median relative abundance in patients with axial SpA after receiving biological treatment for one year. c-d) Alpha and beta diversity analyses, respectively, of axial SpA patients before and after treatment compared to control individuals. Labeled points in d represent group means.Disclosures: v. Rios-Rodriguez, Falk, AbbVie/Abbott; M. Essex, None; J. Rademacher, Novartis, UCB; M. Torgutalp, UCB, AbbVie/Abbott; F. Proft, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, Roche, UCB; U. Löber, None; L. Marko, None; S. Forslund, None; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.

