Back
Poster Session D
Session: (2052–2107) SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
2084: COVID-19 Vaccination in Autoimmune Diseases Study: Vaccine Safety in Systemic Lupus Erythematosus
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Naveen R, DM
Sanjay Gandhi Post graduate institute of medical sciences
Lucknow, Uttar Pradesh, India
Abstract Poster Presenter(s)
Naveen R1, Elena Nikiphorou2, Mrudula Joshi3, Parikshit Sen4, Vishwesh Agarwal5, Sinan Kardes6, James B. Lilleker7, Hector Chinoy8, Oliver Distler9, Minchul Kim10, Ai Lyn Tan11, Samuel Shinjo12, Babur Salim13, Tamer A Gheita14, Nelly Ziade15, Tsvetelina Velikova16, Tulika Chatterjee10, Arvind Nune17, Marcin Milchert18, Abraham Edgar Gracia-Ramos19, Albert Selva O’Callaghan20, Miguel Angel Saavedra Salinas21, Lorenzo Cavagna22, Masataka Kuwana23, Johannes Knitza24, Jessica Day25, Ashima Makol26, Rohit Aggarwal27, Vikas Agarwal1, Latika Gupta28, Lisa S Traboco29, CoVAD Study Group30 and Ioannis Parodis31, 1Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, 2Leiden University Medical Center & King's College London, London, United Kingdom, 3Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals, Pune, India, 4Maulana Azad Medical College, New Delhi, India, 5Mahatma Gandhi Missions Medical College, Lucknow, India, 6Istanbul University, Istanbul, Turkey, 7The University of Manchester, Manchester, United Kingdom, 8The University of Manchester, Sale, United Kingdom, 9Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 10University of Illinois College of Medicine Peoria, Peoria, IL, 11University of Leeds, Leeds, United Kingdom, 12Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil, 13Fauji foundation hospital Rawalpindi, Rawalpindi, Pakistan, 14Rheumatology Department, Faculty of Medicine, Cairo University, Cairo, Egypt, 15Saint-Joseph University, Beirut, Lebanon, 16Sofia University St. Kliment Ohridski, Sofia, Bulgaria, 17Southport and Ormskirk Hospital NHS Trust, Southport, United Kingdom, 18Pomeranian Medical University in Szczecin, Szczecin, Poland, 19Instituto Mexicano del Seguro Social, Ciudad de México, Mexico, 20Hospital Universitari Vall d'Hebron, Barcelona, Spain, 21IMSS, Ciudad de México, Mexico, 22Università di Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, Pavia, Italy, 23Nippon Medical School Graduate School of Medicine, Tokyo, Japan, 24Department of Internal Medicine 3 – Rheumatology and Immunology, Friedrich-Alexander-University Erlangen-Nürnberg and Universitätsklinikum Erlangen, 91054 Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-UniversityErlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany, 25Walter and Eliza Hall Institute, Melbourne, Australia, 26Mayo Clinic, Rochester, MN, Rochester, MN, 27Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, 28Royal Wolverhampton Trust, Wolverhampton/University of Manchester, United Kingdom, 29St Luke's Medical Center - BGC, Manila, Philippines, 30CoVAD study group, Wolwehampton, United Kingdom, 31Karolinska Institutet, Stockholm, Sweden
Background/Purpose: The COVID-19 vaccination in autoimmune diseases (COVAD) study is a large-scale real-world survey on COVID-19 vaccine safety in autoimmune rheumatic diseases (AIRDs), including systemic lupus erythematosus (SLE). The COVAD study aimed to assess adverse events (AEs) related to COVID-19 vaccination up to seven days post-vaccination in SLE patients.
Methods: The COVAD study group comprised >110 collaborators across 94 countries. The study was conducted in March-December 2021. An online survey platform captured self-reported COVID-19 vaccination-related AEs data in SLE, other AIRDs, other non-rheumatic autoimmune diseases (nrAIDs), and healthy controls (HCs). Active and inactive disease was patient self-reported. Descriptive statistics and multivariable regression adjusted for age, gender, ethnicity, vaccine type, and disease modifying anti-rheumatic drugs (DMARDs) were employed. Only odd's ratio significant in regression are reported in the results.
Results: Of the 9462 complete survey respondents, 5.5% (n=583) were SLE patients; those had a mean (SD) age of 40.1 (12) years, and 94.5% were females, 40.5% were Asians, and 71% had received 2 vaccine doses. Pfizer (43.1%) and Oxford/AstraZeneca(11.7%) were the most common vaccines received (Table 1).
Overall, vaccination-related AE were reported by 83.0% of SLE patients (83.0% reported minor and 2.6% major AE). Patients with active and inactive SLE reported similar AE and hospitalization frequencies. Among SLE patients, Pfizer recipients reported higher frequencies of overall AE [OR 2.1 (1.5–2.5), p=0.016], minor AE [OR 2.1 (1.5–2.5), p= 0.016], injection site pain [OR 1.3 (1.04–1.8), p < 0.001], and lower body ache [OR 0.4 (0.2–0.7), p=0.003] compared with recipients of other vaccines. Oxford/AstraZeneca recipients also reported higher frequencies of body ache [OR 2.5 (1.3–4.8), p=0.003], fever [OR 2.7 (1.4-5.2), p=0.002], and chills [OR 3.1 (1.3-6.6), p=0.005] compared with recipients of other vaccines. Moderna recipients reported higher frequencies of body ache, fever, chills, and rashes (OR ranging 2.6 to 4.3; Table 2). Sinopharm recipients reported lower frequencies of injection site pain [OR 0.2 (0.1-0.6), p=0.003]. Hospitalization frequencies were similar across vaccine types.
SLE patients on various DMARDs reported similar AE frequencies, except for less frequent chills reported by hydroxychloroquine users compared with non-users [OR 0.5 (0.3–0.9); Table 3]. When compared with AIRDs, nrAIDs, and HC, SLE patients reported similar overall AE and hospitalization frequencies, yet higher frequencies of rashes [OR 1.2 (1.01–1.5), p=0.038] compared with HC, lower frequencies of chills [OR 0.6 (0.4–0.8), p=0.005 and p=0.003] compared with AIRDs and nrAIDs, and lower frequencies of fatigue [OR 0.6 (0.4–0.9), p=0.020] compared with nrAIDs.
Conclusion: Despite differences in AEs across COVID-19 vaccines, all vaccines were overall well-tolerated in SLE patients. Similar AE frequencies were reported by patients with active and inactive SLE. AE frequencies were similar irrespective of background DMARD. These findings are reassuring with regard to safety of COVID-19 vaccination safety in SLE.
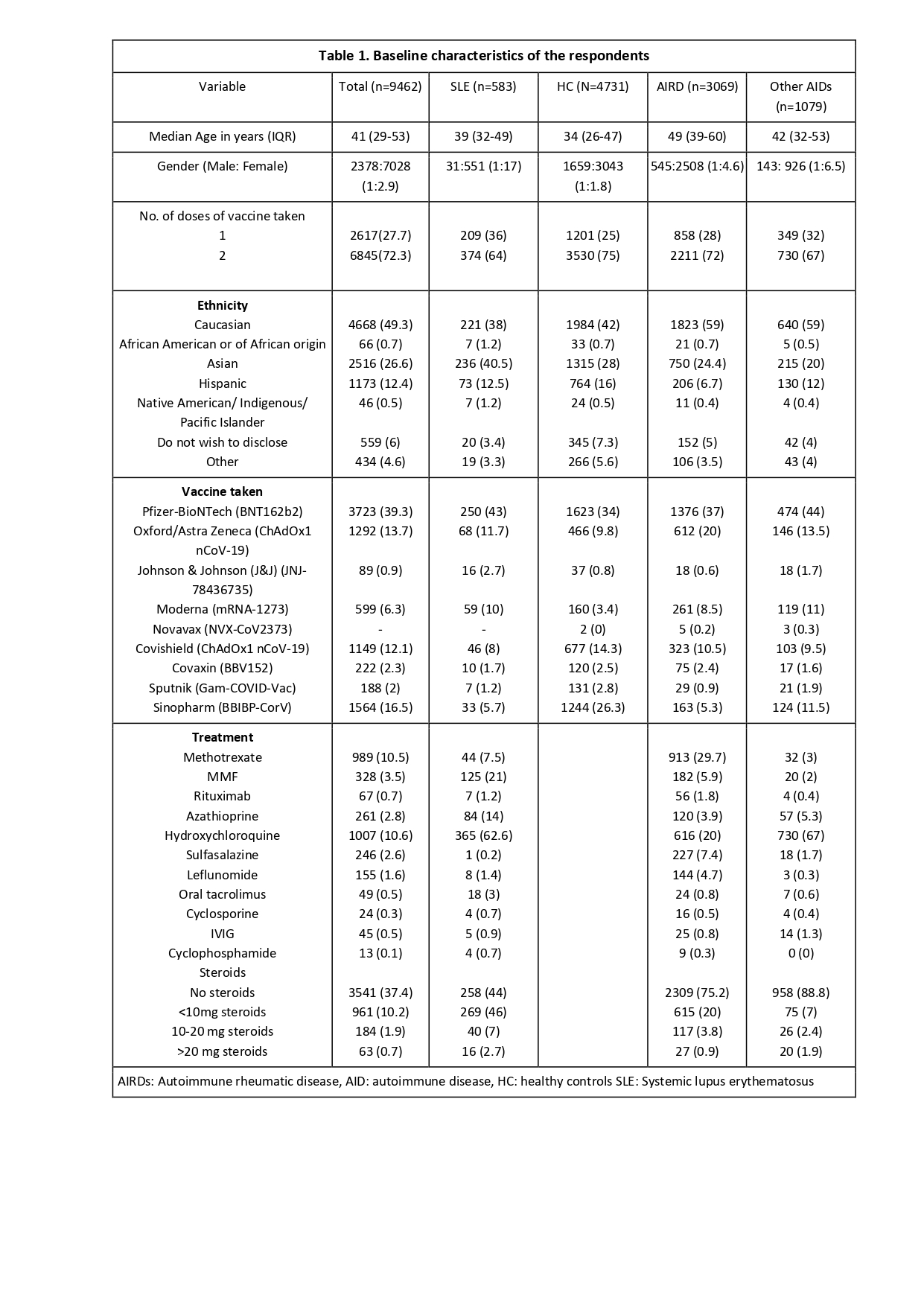 Table 1. Baseline characteristics of the respondents
Table 1. Baseline characteristics of the respondents
.jpg) Table 2. Vaccine related ADE among SLE patients based on the vaccine type
Table 2. Vaccine related ADE among SLE patients based on the vaccine type
.jpg) Table 3. Vaccine related ADE among SLE patients based on the Immunosuppression received
Table 3. Vaccine related ADE among SLE patients based on the Immunosuppression received
Disclosures: N. R, None; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius; M. Joshi, None; P. Sen, None; V. Agarwal, None; S. Kardes, None; J. Lilleker, None; H. Chinoy, Eli Lilly, UCB; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; M. Kim, None; A. Tan, None; S. Shinjo, None; B. Salim, None; T. Gheita, None; N. Ziade, Pfizer, Roche, AbbVie/Abbott, Eli Lilly, Boehringer-Ingelheim, Janssen; T. Velikova, None; T. Chatterjee, None; A. Nune, None; M. Milchert, None; A. Gracia-Ramos, None; A. O’Callaghan, None; M. Saavedra Salinas, GlaxoSmithKlein(GSK), AbbVie/Abbott; L. Cavagna, None; M. Kuwana, Boehringer-Ingelheim, Ono pharmaceuticals, Mochida, AbbVie/Abbott, Astellas, Janssen, Bayer, Corbus, Horizon; J. Knitza, AbbVie, Novartis, ThermoFisher, UCB, ABATON, Sanofi, Medac, Lilly, BMS, Gilead, GSK, Werfen, Vila Health, Böhringer Ingelheim, Janssen, Galapagos, Chugai; J. Day, CSL; A. Makol, Boehringer-Ingelheim; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; V. Agarwal, None; L. Gupta, None; L. Traboco, None; C. Study Group, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche.
Background/Purpose: The COVID-19 vaccination in autoimmune diseases (COVAD) study is a large-scale real-world survey on COVID-19 vaccine safety in autoimmune rheumatic diseases (AIRDs), including systemic lupus erythematosus (SLE). The COVAD study aimed to assess adverse events (AEs) related to COVID-19 vaccination up to seven days post-vaccination in SLE patients.
Methods: The COVAD study group comprised >110 collaborators across 94 countries. The study was conducted in March-December 2021. An online survey platform captured self-reported COVID-19 vaccination-related AEs data in SLE, other AIRDs, other non-rheumatic autoimmune diseases (nrAIDs), and healthy controls (HCs). Active and inactive disease was patient self-reported. Descriptive statistics and multivariable regression adjusted for age, gender, ethnicity, vaccine type, and disease modifying anti-rheumatic drugs (DMARDs) were employed. Only odd's ratio significant in regression are reported in the results.
Results: Of the 9462 complete survey respondents, 5.5% (n=583) were SLE patients; those had a mean (SD) age of 40.1 (12) years, and 94.5% were females, 40.5% were Asians, and 71% had received 2 vaccine doses. Pfizer (43.1%) and Oxford/AstraZeneca(11.7%) were the most common vaccines received (Table 1).
Overall, vaccination-related AE were reported by 83.0% of SLE patients (83.0% reported minor and 2.6% major AE). Patients with active and inactive SLE reported similar AE and hospitalization frequencies. Among SLE patients, Pfizer recipients reported higher frequencies of overall AE [OR 2.1 (1.5–2.5), p=0.016], minor AE [OR 2.1 (1.5–2.5), p= 0.016], injection site pain [OR 1.3 (1.04–1.8), p < 0.001], and lower body ache [OR 0.4 (0.2–0.7), p=0.003] compared with recipients of other vaccines. Oxford/AstraZeneca recipients also reported higher frequencies of body ache [OR 2.5 (1.3–4.8), p=0.003], fever [OR 2.7 (1.4-5.2), p=0.002], and chills [OR 3.1 (1.3-6.6), p=0.005] compared with recipients of other vaccines. Moderna recipients reported higher frequencies of body ache, fever, chills, and rashes (OR ranging 2.6 to 4.3; Table 2). Sinopharm recipients reported lower frequencies of injection site pain [OR 0.2 (0.1-0.6), p=0.003]. Hospitalization frequencies were similar across vaccine types.
SLE patients on various DMARDs reported similar AE frequencies, except for less frequent chills reported by hydroxychloroquine users compared with non-users [OR 0.5 (0.3–0.9); Table 3]. When compared with AIRDs, nrAIDs, and HC, SLE patients reported similar overall AE and hospitalization frequencies, yet higher frequencies of rashes [OR 1.2 (1.01–1.5), p=0.038] compared with HC, lower frequencies of chills [OR 0.6 (0.4–0.8), p=0.005 and p=0.003] compared with AIRDs and nrAIDs, and lower frequencies of fatigue [OR 0.6 (0.4–0.9), p=0.020] compared with nrAIDs.
Conclusion: Despite differences in AEs across COVID-19 vaccines, all vaccines were overall well-tolerated in SLE patients. Similar AE frequencies were reported by patients with active and inactive SLE. AE frequencies were similar irrespective of background DMARD. These findings are reassuring with regard to safety of COVID-19 vaccination safety in SLE.
 Table 1. Baseline characteristics of the respondents
Table 1. Baseline characteristics of the respondents.jpg) Table 2. Vaccine related ADE among SLE patients based on the vaccine type
Table 2. Vaccine related ADE among SLE patients based on the vaccine type.jpg) Table 3. Vaccine related ADE among SLE patients based on the Immunosuppression received
Table 3. Vaccine related ADE among SLE patients based on the Immunosuppression receivedDisclosures: N. R, None; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius; M. Joshi, None; P. Sen, None; V. Agarwal, None; S. Kardes, None; J. Lilleker, None; H. Chinoy, Eli Lilly, UCB; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; M. Kim, None; A. Tan, None; S. Shinjo, None; B. Salim, None; T. Gheita, None; N. Ziade, Pfizer, Roche, AbbVie/Abbott, Eli Lilly, Boehringer-Ingelheim, Janssen; T. Velikova, None; T. Chatterjee, None; A. Nune, None; M. Milchert, None; A. Gracia-Ramos, None; A. O’Callaghan, None; M. Saavedra Salinas, GlaxoSmithKlein(GSK), AbbVie/Abbott; L. Cavagna, None; M. Kuwana, Boehringer-Ingelheim, Ono pharmaceuticals, Mochida, AbbVie/Abbott, Astellas, Janssen, Bayer, Corbus, Horizon; J. Knitza, AbbVie, Novartis, ThermoFisher, UCB, ABATON, Sanofi, Medac, Lilly, BMS, Gilead, GSK, Werfen, Vila Health, Böhringer Ingelheim, Janssen, Galapagos, Chugai; J. Day, CSL; A. Makol, Boehringer-Ingelheim; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; V. Agarwal, None; L. Gupta, None; L. Traboco, None; C. Study Group, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche.

