Back
Ignite Talk
Session: Ignite Session 2B
0177: COVID-19 Vaccination-related Short-term Adverse Events in Patients with Idiopathic Inflammatory Myositis and Autoimmune Multimorbidity: Results from the COVID-19 Vaccination in Autoimmune Diseases Survey
Saturday, November 12, 2022
2:20 PM – 2:25 PM Eastern Time
Location: Center City Stage
- MD
Mrinalini Dey, MRCP, MA, MBBCH
Queen Elizabeth Hospital, London & Institute of Life Course and Medical Sciences, University of Liverpool
London, United KingdomDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Mrinalini Dey1, Naveen R2, Elena Nikiphorou3, Parikshit Sen4, James B. Lilleker5, Vishwesh Agarwal6, Sinan Kardes7, Jessica Day8, Marcin Milchert9, Mrudula Joshi10, Tamer A Gheita11, Babur Salim12, Tsvetelina Velikova13, Abraham Edgar Gracia-Ramos14, Ioannis Parodis15, Albert Selva O’Callaghan16, Minchul Kim17, Tulika Chatterjee17, Ai Lyn Tan18, Ashima Makol19, Arvind Nune20, Lorenzo Cavagna21, Miguel Angel Saavedra Salinas22, Samuel Shinjo23, Nelly Ziade24, Johannes Knitza25, Masataka Kuwana26, Oliver Distler27, Hector Chinoy28, John Pauling29, Chris Wincup30, Vikas Agarwal2, Rohit Aggarwal31 and Latika Gupta32, 1Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom, 2Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, 3Leiden University Medical Center & King's College London, London, United Kingdom, 4Maulana Azad Medical College, New Delhi, India, 5The University of Manchester, Manchester, United Kingdom, 6Mahatma Gandhi Missions Medical College, Lucknow, India, 7Istanbul University, Istanbul, Turkey, 8Walter and Eliza Hall Institute, Melbourne, Australia, 9Pomeranian Medical University in Szczecin, Szczecin, Poland, 10Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals, Pune, India, 11Rheumatology Department, Faculty of Medicine, Cairo University, Cairo, Egypt, 12Fauji foundation hospital Rawalpindi, Rawalpindi, Pakistan, 13Sofia University St. Kliment Ohridski, Sofia, Bulgaria, 14Instituto Mexicano del Seguro Social, Ciudad de México, Mexico, 15Karolinska Institutet, Stockholm, Sweden, 16Hospital Universitari Vall d'Hebron, Barcelona, Spain, 17University of Illinois College of Medicine Peoria, Peoria, IL, 18University of Leeds, Leeds, United Kingdom, 19Mayo Clinic, Rochester, MN, Rochester, MN, 20Southport and Ormskirk Hospital NHS Trust, Southport, United Kingdom, 21Università di Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, Pavia, Italy, 22IMSS, Ciudad de México, Mexico, 23Faculdade de Medicina FMUSP, Universidade de São Paulo, São Paulo, Brazil, 24Saint-Joseph University, Beirut, Lebanon, 25Department of Internal Medicine 3 – Rheumatology and Immunology, Friedrich-Alexander-University Erlangen-Nürnberg and Universitätsklinikum Erlangen, 91054 Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-UniversityErlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany, 26Nippon Medical School Graduate School of Medicine, Tokyo, Japan, 27Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland, 28The University of Manchester, Sale, United Kingdom, 29North Bristol NHS Trust, Bristol, United Kingdom, 30Rayne Institute, University College London, London, United Kingdom, 31Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, 32Royal Wolverhampton Trust, Wolverhampton/University of Manchester, United Kingdom
Background/Purpose: COVID-19 vaccines are safe and effective, though patients with rare rheumatic diseases like idiopathic inflammatory myositis (IIMs), and those with multiple comorbidities continue to be hesitant in taking the vaccine. Adverse events (AEs) after vaccination are not extensively studied in those with multiple coexisting autoimmune diseases. Patients with IIM often have multiple autoimmune rheumatic and autoimmune non-rheumatic comorbidities (IIM-AIDs), with potentially increased risk of AEs. The COVAD study aimed to assess COVID-19 vaccination-related adverse events (AEs) till seven days post-vaccination in IIM-AIDs compared to IIMs and healthy controls (HCs) group.
Methods: The COVAD study group comprised >110 collaborators across 94 countries. The study was conducted from March-December 2021. A survey monkey platform-based self-reported online survey captured data related to COVID-19 vaccination-related AEs in IIMs, AIDs, and HCs. IIM-AIDs patients comprised rheumatic AIDs like overlap syndromes, vasculitis, etc and non-rheumatic AIDs like inflammatory bowel disease, multiple sclerosis, hypothyroidism etc. We compared COVID-19 vaccination-related AEs among IIM-AID patients and IIM alone and HCs, adjusting for age, gender, ethnicity, COVID-19 vaccine type, immunosuppression received, and the numbers of AIDs, using binary logistic regression. Statistically significant results following multivariate regression are reported.
Results: Among 6099 participants, 1387 (22.7%) IIM, 4712 (77.2%) HC, 66.3% females, were included from a total of 18,882 respondents: 573 (41.0%) people with IIM-AIDs; 814 (59.0%) with IIM without other AIDs; and 4712 HCs (Figure 1). People with IIM were older [median age 54 (45-66) IIM-AIDs, 64 (50-73) IIM, 34 (26-47) HC years, p< 0.001]. BNT162b2 (Pfizer)(37.5%) and ChAdOx1 nCoV-19 (Oxford) (11.1%) were the most common vaccines received.
When compared to IIM alone patients, IIM-AID patients reported higher overall AEs [OR 1.5 (1.1-2.1)], minor AE [OR 1.5 (1.1-2.1)] and major AE [OR 3 (1.5-5.8)]. IIM-AIDs patients also reported higher body ache, nausea, headache, and fatigue (OR ranging 1.3-2.3, Table 2). After adjusting for the number of AIDs, the major AEs equalized but overall AEs, and minor AEs, such as fatigue remained higher in IIM-AIDs (Table 2).
When compared to HCs, IIM-AIDs patients reported similar overall AEs, minor AEs but higher major AEs [OR 2 (1.2-3.3)] nausea/vomiting [OR 1.4 (1.01-2)], headache [OR 1.2 (1.01-1.6)], and fatigue [OR 1.3 (1.03-1.6)].
Dermatomyositis (DM) patients with AIDs (n=183) reported higher major AEs [OR 4.3 (1.5-12)] compared to DM alone (n=293).
Active IIM with AIDs (n=482) reported higher overall AEs [OR 1.5 (1.1-2.2)], minor AEs [OR 1.5 (1.1-2.2)] and major AEs [OR 2.6 (1.2-5.2)] compared to active IIM alone (n=643).>
Conclusion: COVID-19 vaccination is safe with minimal to no risks of short-term AEs in patients with IIM without other concomitant autoimmune diseases. The presence of autoimmune multimorbidity conferred higher self-reported short-term risks of overall, major, and minor COVID-19 vaccination-related AEs seven days post-vaccination in IIM patients, particularly in those with active IIM.
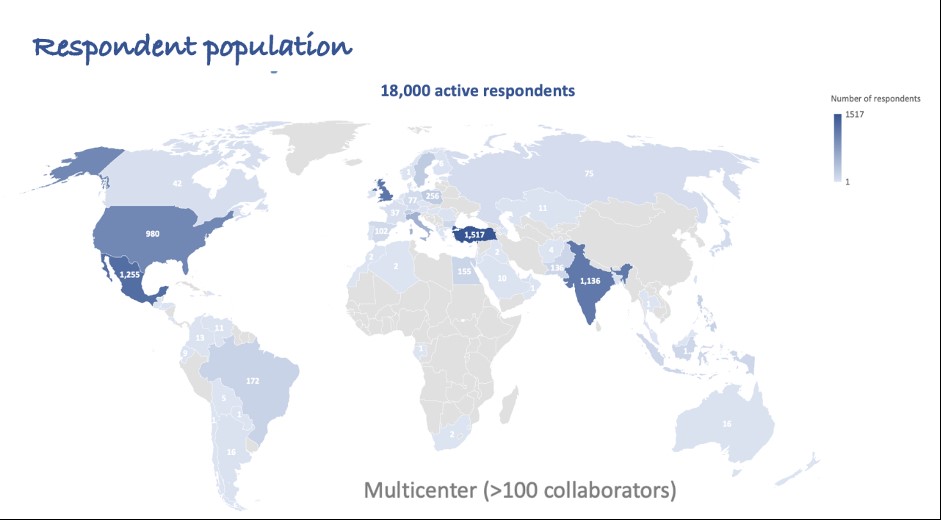 Figure 1: Summary of respondents to COVAD survey
Figure 1: Summary of respondents to COVAD survey
.jpg) Table 1: Basic demographics and characteristics of cohort
Table 1: Basic demographics and characteristics of cohort
.jpg) Table 2. Comparison of vaccine related AE among IIM-AIDs and IIM alone
Table 2. Comparison of vaccine related AE among IIM-AIDs and IIM alone
Disclosures: M. Dey, None; N. R, None; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius; P. Sen, None; J. Lilleker, None; V. Agarwal, None; S. Kardes, None; J. Day, CSL; M. Milchert, None; M. Joshi, None; T. Gheita, None; B. Salim, None; T. Velikova, None; A. Gracia-Ramos, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche; A. O’Callaghan, None; M. Kim, None; T. Chatterjee, None; A. Tan, None; A. Makol, Boehringer-Ingelheim; A. Nune, None; L. Cavagna, None; M. Saavedra Salinas, GlaxoSmithKlein(GSK), AbbVie/Abbott; S. Shinjo, None; N. Ziade, Pfizer, Roche, AbbVie/Abbott, Eli Lilly, Boehringer-Ingelheim, Janssen; J. Knitza, AbbVie, Novartis, ThermoFisher, UCB, ABATON, Sanofi, Medac, Lilly, BMS, Gilead, GSK, Werfen, Vila Health, Böhringer Ingelheim, Janssen, Galapagos, Chugai; M. Kuwana, Boehringer-Ingelheim, Ono pharmaceuticals, Mochida, AbbVie/Abbott, Astellas, Janssen, Bayer, Corbus, Horizon; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; H. Chinoy, Eli Lilly, UCB; J. Pauling, None; C. Wincup, None; V. Agarwal, None; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; L. Gupta, None.
Background/Purpose: COVID-19 vaccines are safe and effective, though patients with rare rheumatic diseases like idiopathic inflammatory myositis (IIMs), and those with multiple comorbidities continue to be hesitant in taking the vaccine. Adverse events (AEs) after vaccination are not extensively studied in those with multiple coexisting autoimmune diseases. Patients with IIM often have multiple autoimmune rheumatic and autoimmune non-rheumatic comorbidities (IIM-AIDs), with potentially increased risk of AEs. The COVAD study aimed to assess COVID-19 vaccination-related adverse events (AEs) till seven days post-vaccination in IIM-AIDs compared to IIMs and healthy controls (HCs) group.
Methods: The COVAD study group comprised >110 collaborators across 94 countries. The study was conducted from March-December 2021. A survey monkey platform-based self-reported online survey captured data related to COVID-19 vaccination-related AEs in IIMs, AIDs, and HCs. IIM-AIDs patients comprised rheumatic AIDs like overlap syndromes, vasculitis, etc and non-rheumatic AIDs like inflammatory bowel disease, multiple sclerosis, hypothyroidism etc. We compared COVID-19 vaccination-related AEs among IIM-AID patients and IIM alone and HCs, adjusting for age, gender, ethnicity, COVID-19 vaccine type, immunosuppression received, and the numbers of AIDs, using binary logistic regression. Statistically significant results following multivariate regression are reported.
Results: Among 6099 participants, 1387 (22.7%) IIM, 4712 (77.2%) HC, 66.3% females, were included from a total of 18,882 respondents: 573 (41.0%) people with IIM-AIDs; 814 (59.0%) with IIM without other AIDs; and 4712 HCs (Figure 1). People with IIM were older [median age 54 (45-66) IIM-AIDs, 64 (50-73) IIM, 34 (26-47) HC years, p< 0.001]. BNT162b2 (Pfizer)(37.5%) and ChAdOx1 nCoV-19 (Oxford) (11.1%) were the most common vaccines received.
When compared to IIM alone patients, IIM-AID patients reported higher overall AEs [OR 1.5 (1.1-2.1)], minor AE [OR 1.5 (1.1-2.1)] and major AE [OR 3 (1.5-5.8)]. IIM-AIDs patients also reported higher body ache, nausea, headache, and fatigue (OR ranging 1.3-2.3, Table 2). After adjusting for the number of AIDs, the major AEs equalized but overall AEs, and minor AEs, such as fatigue remained higher in IIM-AIDs (Table 2).
When compared to HCs, IIM-AIDs patients reported similar overall AEs, minor AEs but higher major AEs [OR 2 (1.2-3.3)] nausea/vomiting [OR 1.4 (1.01-2)], headache [OR 1.2 (1.01-1.6)], and fatigue [OR 1.3 (1.03-1.6)].
Dermatomyositis (DM) patients with AIDs (n=183) reported higher major AEs [OR 4.3 (1.5-12)] compared to DM alone (n=293).
Active IIM with AIDs (n=482) reported higher overall AEs [OR 1.5 (1.1-2.2)], minor AEs [OR 1.5 (1.1-2.2)] and major AEs [OR 2.6 (1.2-5.2)] compared to active IIM alone (n=643).>
Conclusion: COVID-19 vaccination is safe with minimal to no risks of short-term AEs in patients with IIM without other concomitant autoimmune diseases. The presence of autoimmune multimorbidity conferred higher self-reported short-term risks of overall, major, and minor COVID-19 vaccination-related AEs seven days post-vaccination in IIM patients, particularly in those with active IIM.
 Figure 1: Summary of respondents to COVAD survey
Figure 1: Summary of respondents to COVAD survey.jpg) Table 1: Basic demographics and characteristics of cohort
Table 1: Basic demographics and characteristics of cohort.jpg) Table 2. Comparison of vaccine related AE among IIM-AIDs and IIM alone
Table 2. Comparison of vaccine related AE among IIM-AIDs and IIM aloneDisclosures: M. Dey, None; N. R, None; E. Nikiphorou, Pfizer, Celltrion, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Fresenius; P. Sen, None; J. Lilleker, None; V. Agarwal, None; S. Kardes, None; J. Day, CSL; M. Milchert, None; M. Joshi, None; T. Gheita, None; B. Salim, None; T. Velikova, None; A. Gracia-Ramos, None; I. Parodis, GlaxoSmithKlein(GSK), Amgen, AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, Gilead, Janssen, Novartis, Roche; A. O’Callaghan, None; M. Kim, None; T. Chatterjee, None; A. Tan, None; A. Makol, Boehringer-Ingelheim; A. Nune, None; L. Cavagna, None; M. Saavedra Salinas, GlaxoSmithKlein(GSK), AbbVie/Abbott; S. Shinjo, None; N. Ziade, Pfizer, Roche, AbbVie/Abbott, Eli Lilly, Boehringer-Ingelheim, Janssen; J. Knitza, AbbVie, Novartis, ThermoFisher, UCB, ABATON, Sanofi, Medac, Lilly, BMS, Gilead, GSK, Werfen, Vila Health, Böhringer Ingelheim, Janssen, Galapagos, Chugai; M. Kuwana, Boehringer-Ingelheim, Ono pharmaceuticals, Mochida, AbbVie/Abbott, Astellas, Janssen, Bayer, Corbus, Horizon; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; H. Chinoy, Eli Lilly, UCB; J. Pauling, None; C. Wincup, None; V. Agarwal, None; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech; L. Gupta, None.

