Back
Ignite Talk
Session: Ignite Session 1C
0649: Phosphofructokinase P Fine-Tunes T Regulatory Cell Metabolism, Function and Stability in Systemic Autoimmunity
Saturday, November 12, 2022
1:35 PM – 1:40 PM Eastern Time
Location: South Philly Stage
- MS
Marc Scherlinger, MD, PhD
Beth Israel Deaconess Medical Center Harvard University
Strasbourg, FranceDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Marc Scherlinger1, Wenliang Pan2, Ryo Hisada1, Milena Vukelic3, Masataka Umeda1, Afroditi Boulougoura2, Maria Tsokos1 and George Tsokos2, 1BIDMC Harvard University, Boston, MA, 2Division of Rheumatology and Clinical Immunology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, 3Albert Einstein College of Medicine, New York, NY
Background/Purpose: Systemic lupus erythematosus (SLE) is characterized by a defective T regulatory (Treg) cell compartment which participate in immune dysregulation. Although the underlying mechanism are unknown, dysregulated T cell metabolism has been widely reported in SLE, and its normalization leads to disease improvement (1). Calcium/calmodulin dependent protein kinase (CaMK4) activity is increased in SLE and has been shown to affect T cell metabolism (2). Our objective was to investigate the mechanisms underlying Treg cell metabolic dysregulation in SLE, with a focus on CaMK4.
Methods: We harvested CD62L+CD4+ T cells from wild-type (WT) or Camk4-/- mice and differentiated them in vitro into inducible Treg (iTreg) cells. We evaluated iTreg metabolism using Seahorse XF analyzer and mass spectrometry (metabolomics). Phosphofructokinase activity was assessed by a colorimetric assay (Abcam). In vitro gene knockdown was conducted by transfecting a guide RNA (gRNA) in CRISPR/Cas9-expressing T cells. Treg cell function was evaluated by in vitro immunosuppressive assay and in vivo by the adoptive transfer of T conventional T and iTreg cells (8:1 ratio) in Rag1-/- mice to induce inflammatory colitis. The relevance of CaMK4 in SLE was evaluated in vivo using a T-cell specific knockdown of CaMK4 in the B6.lpr mouse model, and in humans by culturing SLE patient T cells with KN-93, a CaMK4 specific inhibitor.
Results: iTreg cells from Camk4-/- mice had decreased glycolysis and increased mitochondrial metabolism compared to WT mice. Metabolomics studies suggested decreased activity of the rate-limiting glycolysis enzyme phosphofructokinase platelet-type (PFKP). While PFKP mRNA and protein levels were similar between WT and Camk4-/- iTreg, PFKP activity was significantly decreased in Camk4-/- iTreg, suggesting a post-transcriptional control of PFKP activity by CaMK4. Mechanistically, immunoprecipitation experiments confirmed that CaMK4 interacted with PFKP, and phosphoproteomic study suggested that CaMK4 phosphorylated serine residue 539 of PFKP, a site known to control PFKP activity. To confirm the importance of PFKP in Treg biology, we showed that PFKP knockdown significantly improved iTreg function in vitro (p < 0.01) and in vivo using an adoptive colitis model (Fig. 1A-B). In vivo, iTreg lacking PFKP were less likely to lose FoxP3 expression (Fig. 1C) and to produce IL-17A, demonstrating higher Treg stability in an inflammatory environment. On a translational basis, lupus-prone B6.lpr mice with a T-cell specific CaMK4 knockdown displayed improved disease pathology. Human SLE CD4+ T cells had higher PFKP activity compared to healthy donors, and PFKP activity correlated with the SLE disease activity index (SLEDAI, r = 0.579, p < 0.005; Fig. 2A-B). Finally, culture of SLE CD4+ T cells with KN-93 led to a significant decrease in PFKP activity (p < 0.001, Fig. 2C), and an improvement of Treg cell immunosuppressive activity (Fig. 2D-E).
Conclusion: By fine-tuning their immunometabolism, PFKP controls the immunosuppressive function and stability of Treg cell in SLE, and represents a promising therapeutic target.
References:
1. Yin Y, et al. Sci Transl Med. 7(274):274ra18.
2. Kono M, et al. JCI Insight 4(12):e127395.
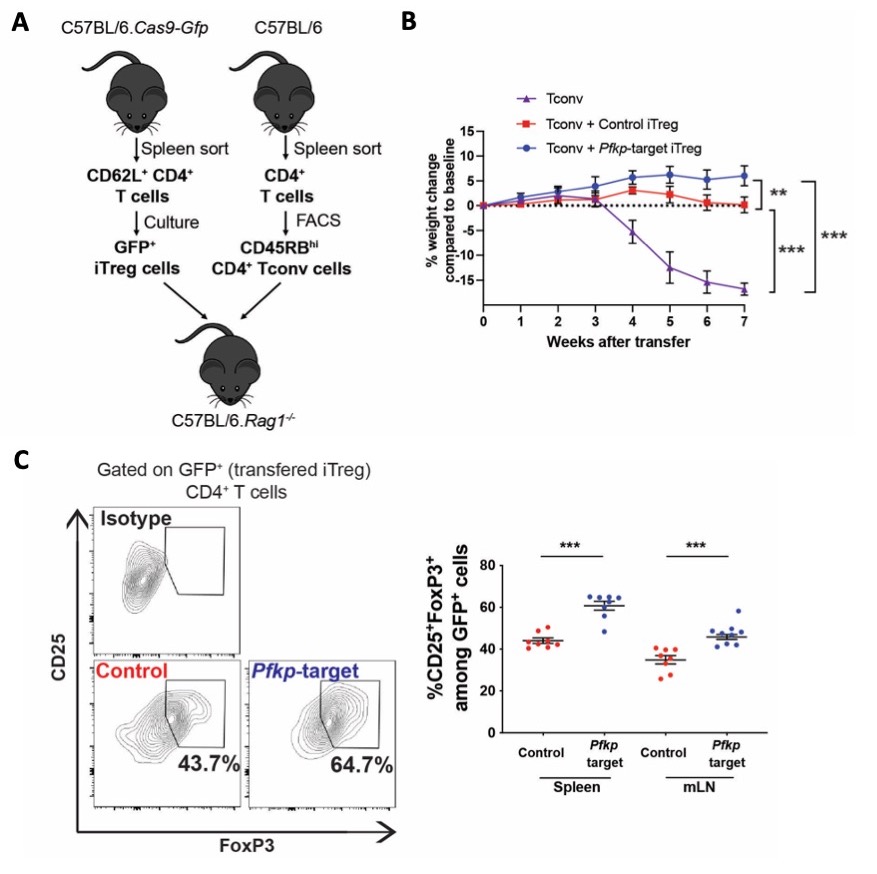 Figure 1: PFKP modulation improves the iTreg cell in vivo function and stability in the adoptive transfer colitis model.
Figure 1: PFKP modulation improves the iTreg cell in vivo function and stability in the adoptive transfer colitis model.
(A) Design of the in vivo experiment. Cas9-GFP expressing CD62L+ CD4+ T cells were transfected with control of Pfkp-target sgRNA and differentiated into iTreg cells. CD45RBhi CD4+ T (Tconv) cells were sorted using FACS from CD4+ T cells presorted from the splenocytes of C57Bl/6 mice. T-deficient RAG1-/- mice were transferred with 4x106 Tconv and 5x105 iTreg. Data are from 3 independent experiments of 2-3 mice per condition. (B) Weight of mice transferred with Tconv cells only (purple, n = 4), Tconv and control iTreg cells (red, n = 8) or Tconv and Pfkp-target iTreg cells (blue, n = 8). After sacrifice, the percentage of CD25+FoxP3+ Treg cells was evaluated among initially transferred iTreg identified as GFP+ CD4+ T cells, in the spleen and mesenteric lymph nodes (mLN; n = 8). Points and bars represent an individual value and s.e.m., respectively. **, p < 0.01; ***, p < 0.001.
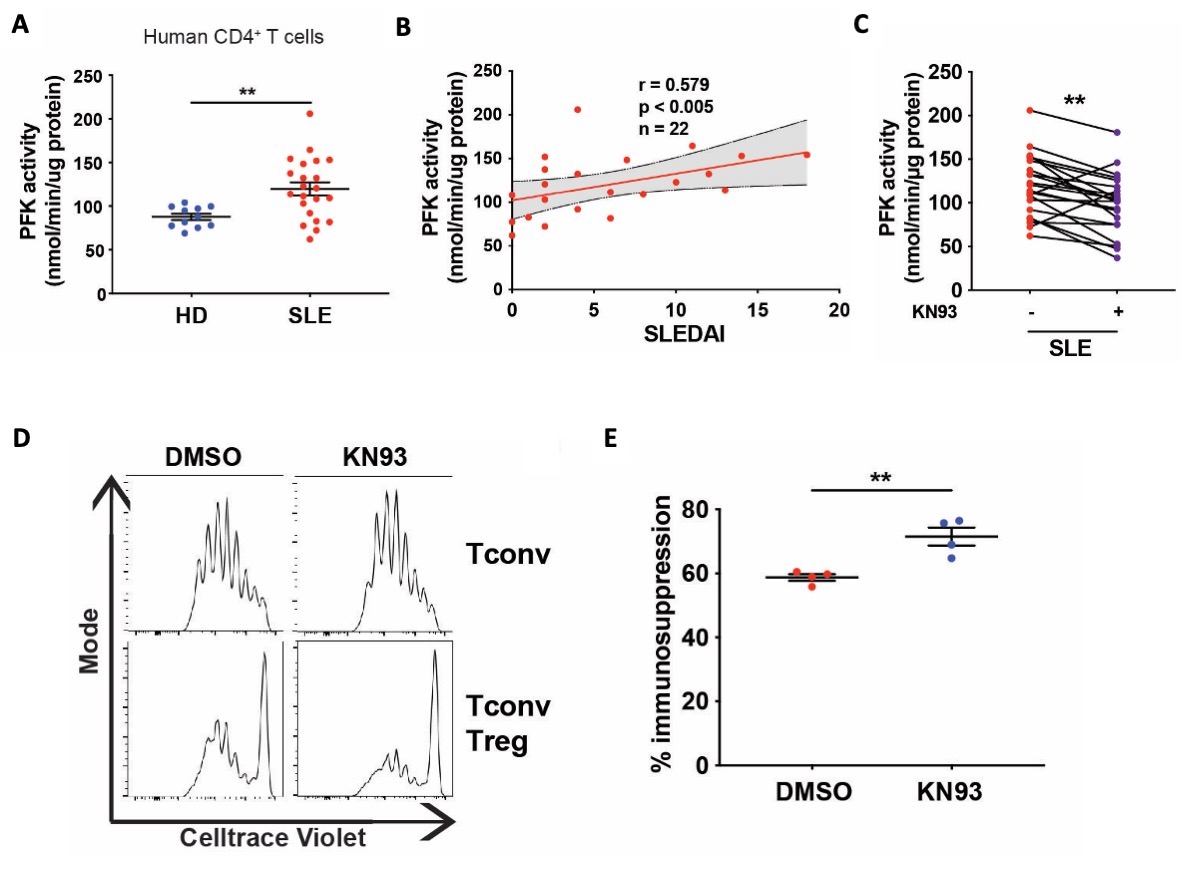 Figure 2: CaMK4 regulates PFKP activity in SLE CD4+ T cells and impairs human Treg cell immunosuppressive functions.
Figure 2: CaMK4 regulates PFKP activity in SLE CD4+ T cells and impairs human Treg cell immunosuppressive functions.
(A) PFK activity was measured from the lysate of CD4+ T cells cultured during 24 hours with CD3/CD28 activation. Samples from HD (n = 11) and SLE patients (n = 22). (B) Spearman correlation of the CD4+ T cells PFK activity and the SLE disease activity index (SLEDAI; n = 22).The delimited grey area indicates the 95% confidence interval of the linear regression. (C) PFK activity was measured from the lysate of CD4+ T cells cultured during 24 hours with CD3/CD28 activation with or without KN93 (10μM; n = 22). (D) Representative results of Tconv cells proliferation. (F) The percentage of immunosuppression was calculated by comparing Treg/Tconv cell proliferation to the Tconv cell proliferation from the corresponding condition (n = 4 biological replicates). Points and bars represent an individual value and s.e.m., respectively. **, p < 0.01.
Disclosures: M. Scherlinger, Sandoz, Amgen, Nordic Pharma; W. Pan, None; R. Hisada, None; M. Vukelic, None; M. Umeda, None; A. Boulougoura, None; M. Tsokos, None; G. Tsokos, None.
Background/Purpose: Systemic lupus erythematosus (SLE) is characterized by a defective T regulatory (Treg) cell compartment which participate in immune dysregulation. Although the underlying mechanism are unknown, dysregulated T cell metabolism has been widely reported in SLE, and its normalization leads to disease improvement (1). Calcium/calmodulin dependent protein kinase (CaMK4) activity is increased in SLE and has been shown to affect T cell metabolism (2). Our objective was to investigate the mechanisms underlying Treg cell metabolic dysregulation in SLE, with a focus on CaMK4.
Methods: We harvested CD62L+CD4+ T cells from wild-type (WT) or Camk4-/- mice and differentiated them in vitro into inducible Treg (iTreg) cells. We evaluated iTreg metabolism using Seahorse XF analyzer and mass spectrometry (metabolomics). Phosphofructokinase activity was assessed by a colorimetric assay (Abcam). In vitro gene knockdown was conducted by transfecting a guide RNA (gRNA) in CRISPR/Cas9-expressing T cells. Treg cell function was evaluated by in vitro immunosuppressive assay and in vivo by the adoptive transfer of T conventional T and iTreg cells (8:1 ratio) in Rag1-/- mice to induce inflammatory colitis. The relevance of CaMK4 in SLE was evaluated in vivo using a T-cell specific knockdown of CaMK4 in the B6.lpr mouse model, and in humans by culturing SLE patient T cells with KN-93, a CaMK4 specific inhibitor.
Results: iTreg cells from Camk4-/- mice had decreased glycolysis and increased mitochondrial metabolism compared to WT mice. Metabolomics studies suggested decreased activity of the rate-limiting glycolysis enzyme phosphofructokinase platelet-type (PFKP). While PFKP mRNA and protein levels were similar between WT and Camk4-/- iTreg, PFKP activity was significantly decreased in Camk4-/- iTreg, suggesting a post-transcriptional control of PFKP activity by CaMK4. Mechanistically, immunoprecipitation experiments confirmed that CaMK4 interacted with PFKP, and phosphoproteomic study suggested that CaMK4 phosphorylated serine residue 539 of PFKP, a site known to control PFKP activity. To confirm the importance of PFKP in Treg biology, we showed that PFKP knockdown significantly improved iTreg function in vitro (p < 0.01) and in vivo using an adoptive colitis model (Fig. 1A-B). In vivo, iTreg lacking PFKP were less likely to lose FoxP3 expression (Fig. 1C) and to produce IL-17A, demonstrating higher Treg stability in an inflammatory environment. On a translational basis, lupus-prone B6.lpr mice with a T-cell specific CaMK4 knockdown displayed improved disease pathology. Human SLE CD4+ T cells had higher PFKP activity compared to healthy donors, and PFKP activity correlated with the SLE disease activity index (SLEDAI, r = 0.579, p < 0.005; Fig. 2A-B). Finally, culture of SLE CD4+ T cells with KN-93 led to a significant decrease in PFKP activity (p < 0.001, Fig. 2C), and an improvement of Treg cell immunosuppressive activity (Fig. 2D-E).
Conclusion: By fine-tuning their immunometabolism, PFKP controls the immunosuppressive function and stability of Treg cell in SLE, and represents a promising therapeutic target.
References:
1. Yin Y, et al. Sci Transl Med. 7(274):274ra18.
2. Kono M, et al. JCI Insight 4(12):e127395.
 Figure 1: PFKP modulation improves the iTreg cell in vivo function and stability in the adoptive transfer colitis model.
Figure 1: PFKP modulation improves the iTreg cell in vivo function and stability in the adoptive transfer colitis model.(A) Design of the in vivo experiment. Cas9-GFP expressing CD62L+ CD4+ T cells were transfected with control of Pfkp-target sgRNA and differentiated into iTreg cells. CD45RBhi CD4+ T (Tconv) cells were sorted using FACS from CD4+ T cells presorted from the splenocytes of C57Bl/6 mice. T-deficient RAG1-/- mice were transferred with 4x106 Tconv and 5x105 iTreg. Data are from 3 independent experiments of 2-3 mice per condition. (B) Weight of mice transferred with Tconv cells only (purple, n = 4), Tconv and control iTreg cells (red, n = 8) or Tconv and Pfkp-target iTreg cells (blue, n = 8). After sacrifice, the percentage of CD25+FoxP3+ Treg cells was evaluated among initially transferred iTreg identified as GFP+ CD4+ T cells, in the spleen and mesenteric lymph nodes (mLN; n = 8). Points and bars represent an individual value and s.e.m., respectively. **, p < 0.01; ***, p < 0.001.
 Figure 2: CaMK4 regulates PFKP activity in SLE CD4+ T cells and impairs human Treg cell immunosuppressive functions.
Figure 2: CaMK4 regulates PFKP activity in SLE CD4+ T cells and impairs human Treg cell immunosuppressive functions. (A) PFK activity was measured from the lysate of CD4+ T cells cultured during 24 hours with CD3/CD28 activation. Samples from HD (n = 11) and SLE patients (n = 22). (B) Spearman correlation of the CD4+ T cells PFK activity and the SLE disease activity index (SLEDAI; n = 22).The delimited grey area indicates the 95% confidence interval of the linear regression. (C) PFK activity was measured from the lysate of CD4+ T cells cultured during 24 hours with CD3/CD28 activation with or without KN93 (10μM; n = 22). (D) Representative results of Tconv cells proliferation. (F) The percentage of immunosuppression was calculated by comparing Treg/Tconv cell proliferation to the Tconv cell proliferation from the corresponding condition (n = 4 biological replicates). Points and bars represent an individual value and s.e.m., respectively. **, p < 0.01.
Disclosures: M. Scherlinger, Sandoz, Amgen, Nordic Pharma; W. Pan, None; R. Hisada, None; M. Vukelic, None; M. Umeda, None; A. Boulougoura, None; M. Tsokos, None; G. Tsokos, None.

