Back
Poster Session D
Sjögren's syndrome
Session: (2017–2051) Sjögren's Syndrome – Basic and Clinical Science Poster
2029: Distinctive Clinical Profiles Associated with anti-SSA/Ro60, anti-Ro52/TRIM21 and Anti-SSB/La Reactivity
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- ip
isabelle peene, MD, PhD
University Hospital Ghent
Ghent, Belgium
Abstract Poster Presenter(s)
Liselotte Deroo1, Helena Achten2, Kristel De Boeck2, Eva Genbrugge2, Wouter Bauters2, Dimitri Roels2, Frederick Dochy2, David Creytens2, Filip Van den bosch3, Dirk Elewaut4 and isabelle peene5, 1Ghent University, Gent, Belgium, 2Ghent University Hospital, Gent, Belgium, 3Department of Internal Medicine and Paediatrics, Ghent University and VIB Centre for Inflammation Research, Ghent, Belgium, 4Department of Rheumatology, Ghent University Hospital, Belgium, VIB-UGent Center for Inflammation Research, Ghent University, Heusden, Belgium, 5Ghent University Hospital, Ghent, Belgium
Background/Purpose: Presence of anti-SSA/Ro antibodies is one of the 2016 ACR-EULAR classification criteria for primary Sjögren's Syndrome (pSS). Anti-SSA/Ro antibodies comprise reactivity against Ro52 and/or Ro60. Although that detection of anti-SSB/La antibodies is no classification criterium anymore, they are present in 23-52% of pSS patients, most frequently together with anti-SSA/Ro antibodies. The value of separate anti-Ro52, anti-Ro60 and anti-SSB/La detection in relation to pSS diagnosis and phenotypical features has not been extensively studied. This study aimed to explore disease characteristics of anti-SSA/Ro positive, suspected and definite pSS patients, in relation to serological profiles based on anti-Ro52, anti-Ro60 and anti-SSB/La reactivity.
Methods: Data of 187 anti-SSA/Ro positive patients included in the Belgian Sjögren's Syndrome Transition Trial (BeSSTT) were analyzed. Of those, 151 were considered definite pSS patients, due to fulfilment of the 2016 ACR-EULAR classification criteria, and 36 suspected, due to reactivity against SSA/Ro without presence of other criteria. None of the patients met any of the ACR-EULAR exclusion criteria for pSS. Patient groups were based on the presence of anti-Ro52, anti-Ro60 and anti-SSB/La antibodies.
Results: Reactivity against isolated Ro60, isolated Ro52, Ro52/Ro60 (double) and Ro52/Ro60 in combination with SSB (triple) was detected in respectively 30, 23, 70 and 60 patients. Mono-anti-Ro60 positive patients showed least pSS features. Mono-anti-Ro52 positive patients reported a significantly higher dryness burden (p=0.016) and tended toward more salivary gland ultrasound (SGUS) abnormalities (p=0.054) than mono-anti-Ro60 positives. Double positive patients showed similar characteristics as mono-anti-Ro52 positive patients, whereas triple positive patients showed more extreme phenotypes with lowest unstimulated salivary flow rates (p=0.002) and Schirmer tests (p=0.002), highest ocular staining scores (p< 0.001), most positive labial salivary gland biopsies (p=0.039), most laboratory abnormalities compatible with B-cell hyperactivity and highest SGUS scores (p< 0.001).
Conclusion: These data indicate that separate detection of anti-Ro52, anti-Ro60 and anti-SSB/La reactivity is not only relevant towards pSS diagnosis, but markedly aids in patient stratification and evaluation of disease burden. In this context, our results point to a stepwise model in which mono-reactivity against Ro60 displayed the least pSS features, whereas these were most present in patients showing triple reactivity against Ro60, Ro52 and SSB/La.
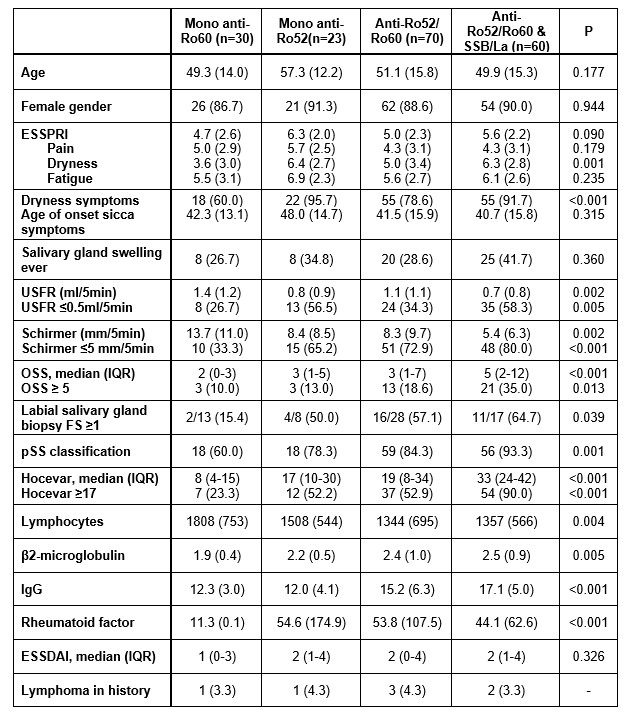 Table 1 Clinical and laboratory parameters amongst serology-based patient groups. Continuous data presented as mean±SD and categorical data as number of patients (%) unless otherwise indicated. P-values refer to comparison of endotypes amongst definite and suspected pSS subgroups, based on Kruskal-Wallis or Chi-squared tests. Abbreviations: ESSPRI=EULAR Sjögren's syndrome patient reported index; USFR=unstimulated salivary flow rate; OSS=ocular staining score; FS=focus score; ESSDAI=EULAR Sjögren's syndrome disease activity index.
Table 1 Clinical and laboratory parameters amongst serology-based patient groups. Continuous data presented as mean±SD and categorical data as number of patients (%) unless otherwise indicated. P-values refer to comparison of endotypes amongst definite and suspected pSS subgroups, based on Kruskal-Wallis or Chi-squared tests. Abbreviations: ESSPRI=EULAR Sjögren's syndrome patient reported index; USFR=unstimulated salivary flow rate; OSS=ocular staining score; FS=focus score; ESSDAI=EULAR Sjögren's syndrome disease activity index.
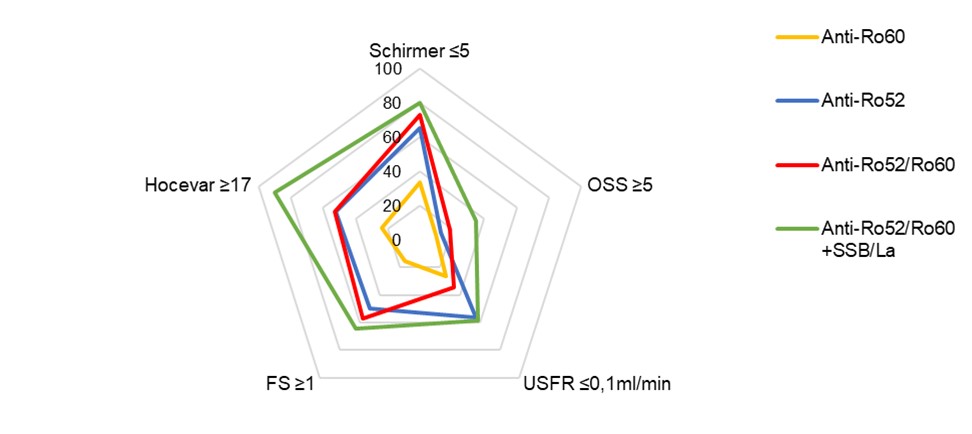 Figure 1 Radar chart illustration pSS key features amongst serological profiles
Figure 1 Radar chart illustration pSS key features amongst serological profiles
Radar chart illustrates prevalence in percentage of key phenotypical features related to pSS amongst patient groups defined by presence of anti-Ro60, anti-Ro52 and anti-SSB/La antibodies. Abbreviations: OSS=ocular staining score; USFR=unstimulated salivary flow rate; FS=focus score.
Disclosures: L. Deroo, None; H. Achten, None; K. De Boeck, None; E. Genbrugge, None; W. Bauters, None; D. Roels, None; F. Dochy, None; D. Creytens, None; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; D. Elewaut, AbbVie, Eli Lilly, Galapagos, Novartis, UCB Pharma; i. peene, None.
Background/Purpose: Presence of anti-SSA/Ro antibodies is one of the 2016 ACR-EULAR classification criteria for primary Sjögren's Syndrome (pSS). Anti-SSA/Ro antibodies comprise reactivity against Ro52 and/or Ro60. Although that detection of anti-SSB/La antibodies is no classification criterium anymore, they are present in 23-52% of pSS patients, most frequently together with anti-SSA/Ro antibodies. The value of separate anti-Ro52, anti-Ro60 and anti-SSB/La detection in relation to pSS diagnosis and phenotypical features has not been extensively studied. This study aimed to explore disease characteristics of anti-SSA/Ro positive, suspected and definite pSS patients, in relation to serological profiles based on anti-Ro52, anti-Ro60 and anti-SSB/La reactivity.
Methods: Data of 187 anti-SSA/Ro positive patients included in the Belgian Sjögren's Syndrome Transition Trial (BeSSTT) were analyzed. Of those, 151 were considered definite pSS patients, due to fulfilment of the 2016 ACR-EULAR classification criteria, and 36 suspected, due to reactivity against SSA/Ro without presence of other criteria. None of the patients met any of the ACR-EULAR exclusion criteria for pSS. Patient groups were based on the presence of anti-Ro52, anti-Ro60 and anti-SSB/La antibodies.
Results: Reactivity against isolated Ro60, isolated Ro52, Ro52/Ro60 (double) and Ro52/Ro60 in combination with SSB (triple) was detected in respectively 30, 23, 70 and 60 patients. Mono-anti-Ro60 positive patients showed least pSS features. Mono-anti-Ro52 positive patients reported a significantly higher dryness burden (p=0.016) and tended toward more salivary gland ultrasound (SGUS) abnormalities (p=0.054) than mono-anti-Ro60 positives. Double positive patients showed similar characteristics as mono-anti-Ro52 positive patients, whereas triple positive patients showed more extreme phenotypes with lowest unstimulated salivary flow rates (p=0.002) and Schirmer tests (p=0.002), highest ocular staining scores (p< 0.001), most positive labial salivary gland biopsies (p=0.039), most laboratory abnormalities compatible with B-cell hyperactivity and highest SGUS scores (p< 0.001).
Conclusion: These data indicate that separate detection of anti-Ro52, anti-Ro60 and anti-SSB/La reactivity is not only relevant towards pSS diagnosis, but markedly aids in patient stratification and evaluation of disease burden. In this context, our results point to a stepwise model in which mono-reactivity against Ro60 displayed the least pSS features, whereas these were most present in patients showing triple reactivity against Ro60, Ro52 and SSB/La.
 Table 1 Clinical and laboratory parameters amongst serology-based patient groups. Continuous data presented as mean±SD and categorical data as number of patients (%) unless otherwise indicated. P-values refer to comparison of endotypes amongst definite and suspected pSS subgroups, based on Kruskal-Wallis or Chi-squared tests. Abbreviations: ESSPRI=EULAR Sjögren's syndrome patient reported index; USFR=unstimulated salivary flow rate; OSS=ocular staining score; FS=focus score; ESSDAI=EULAR Sjögren's syndrome disease activity index.
Table 1 Clinical and laboratory parameters amongst serology-based patient groups. Continuous data presented as mean±SD and categorical data as number of patients (%) unless otherwise indicated. P-values refer to comparison of endotypes amongst definite and suspected pSS subgroups, based on Kruskal-Wallis or Chi-squared tests. Abbreviations: ESSPRI=EULAR Sjögren's syndrome patient reported index; USFR=unstimulated salivary flow rate; OSS=ocular staining score; FS=focus score; ESSDAI=EULAR Sjögren's syndrome disease activity index. Figure 1 Radar chart illustration pSS key features amongst serological profiles
Figure 1 Radar chart illustration pSS key features amongst serological profilesRadar chart illustrates prevalence in percentage of key phenotypical features related to pSS amongst patient groups defined by presence of anti-Ro60, anti-Ro52 and anti-SSB/La antibodies. Abbreviations: OSS=ocular staining score; USFR=unstimulated salivary flow rate; FS=focus score.
Disclosures: L. Deroo, None; H. Achten, None; K. De Boeck, None; E. Genbrugge, None; W. Bauters, None; D. Roels, None; F. Dochy, None; D. Creytens, None; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; D. Elewaut, AbbVie, Eli Lilly, Galapagos, Novartis, UCB Pharma; i. peene, None.

