Back
Ignite Talk
Session: Ignite Session 2A
0075: Real-World Treatment Patterns Among Patients with Connective Tissue Disorder–Related Pulmonary Arterial Hypertension in the United States: A Retrospective Claims-Based Analysis
Saturday, November 12, 2022
2:45 PM – 2:50 PM Eastern Time
Location: Northern Liberties Stage
- YT
Yuen Tsang, PharmD
Janssen Scientific Affairs
Sacramento, CA, United StatesDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Yuen Tsang1, Marjolaine Gauthier-Loiselle2, Vienica Funtanilla3, Hayley Germack3, Ameur Manceur4, Stephanie Liu2, Martin Cloutier2, Patrick Lefebvre2 and Sumeet Panjabi5, 1Janssen Scientific Affairs, LLC., Sacramento, CA, 2Analysis Group, Inc., Montréal, QC, Canada, 3Janssen Scientific Affairs, LLC, Titusville, NJ, 4Analysis Group, Inc, Boston, MA, 5Analysis Group, Inc., Titusville, NB, Canada
Background/Purpose: Connective tissue disorders (CTDs) are the most frequent diseases associated with pulmonary arterial hypertension (PAH), accounting for 11% – 28% of PAH cases. A better understanding of real-world treatment patterns could help identify potential areas for improvement in the management of CTD-related PAH; thus, this study assessed PAH treatment patterns in this population in the US.
Methods: Adult patients initiated on a PAH treatment (index date: 1st initiation date) were identified from Optum Clinformatics® Data Mart Databases (10/01/2015-09/30/2021). Patients had ≥1 inpatient or ≥2 outpatient PAH diagnosis claims and ≥6 months of continuous insurance eligibility without PAH treatment before the index date (i.e., baseline period). Eligible patients were categorized into mutually exclusive cohorts: (1) CTD+PAH: had ≥1 inpatient or ≥2 outpatient CTD diagnosis claims, with ≥1 claim before/on the index date; or (2) PAH: no CTD diagnosis at any time during baseline or follow-up. Treatment patterns were assessed from the index date to the earliest of death, end of continuous insurance eligibility, or end of data availability. A treatment regimen was defined as all PAH agents observed within 60 days of the 1st agent; a ≥90-day gap with no PAH agents indicated treatment discontinuation. Kaplan-Meier (KM) analysis of persistence (time to 1st treatment change) and time to combination therapy was performed.
Results: Among 728 patients in the CTD+PAH cohort and 4,023 patients in the PAH cohort, average follow-up was 18.8 and 19.6 months, respectively. Mean age was 64.2 and 69.1 years in the CTD+PAH and PAH cohorts, with 85.7% and 60.8% female, respectively. For both cohorts, the most common 1st treatment regimens observed were sildenafil (CTD+PAH: 38.7%; PAH: 51.5%), tadalafil (10.0%; 9.4%), and macitentan (8.1%; 5.4%) in monotherapy. Sildenafil, tadalafil, and macitentan were also the most frequent agents included in any of the first 3 treatment regimens (Figure 1). Combination therapy was more frequent in the CTD+PAH cohort than in the PAH cohort (any regimen: 40.9% vs 27.2%; 1st treatment regimen: 26.9% vs 18.5%; 2nd: 52.8% vs 42.0%; 3rd: 55.2% vs 48.5%; Figure 1). KM analysis showed that patients in the CTD+PAH cohort were more likely to be initiated on combination therapy at any time post-index (Figure 2). The vast majority of patients had cardiologist, pulmonologist, and/or rheumatologist visits, but time to combination therapy was similar regardless of the type of specialist visits. Treatment persistence was similar across cohorts and first 3 treatment regimens, with persistence rates ranging from 42.6% to 49.7% at 12 months (Figure 3).
Conclusion: Compared to PAH patients, CTD+PAH patients were younger and more likely to be female. Treatment patterns were generally similar between the CTD+PAH and PAH cohorts, though combination therapy was more frequent in the CTD+PAH cohort. Both cohorts may benefit from broader use of all available PAH treatment classes, including combination therapy to target the nitric oxide, endothelin, and prostacyclin pathways. Our findings also highlight the need to understand low persistence rates with PAH therapies.
.jpg) Abbreviations: CTD: connective tissue disorder; ERA: endothelin receptor antagonist; PAH: pulmonary arterial hypertension; PDE5i: phosphodiesterase type 5 inhibitor; PPA, prostacyclin pathway agent; SGC: soluble guacylate cyclase inhibitor.
Abbreviations: CTD: connective tissue disorder; ERA: endothelin receptor antagonist; PAH: pulmonary arterial hypertension; PDE5i: phosphodiesterase type 5 inhibitor; PPA, prostacyclin pathway agent; SGC: soluble guacylate cyclase inhibitor.
Note:
[1] All agents received are listed regardless of whether they were used as monotherapy or combination therapy.
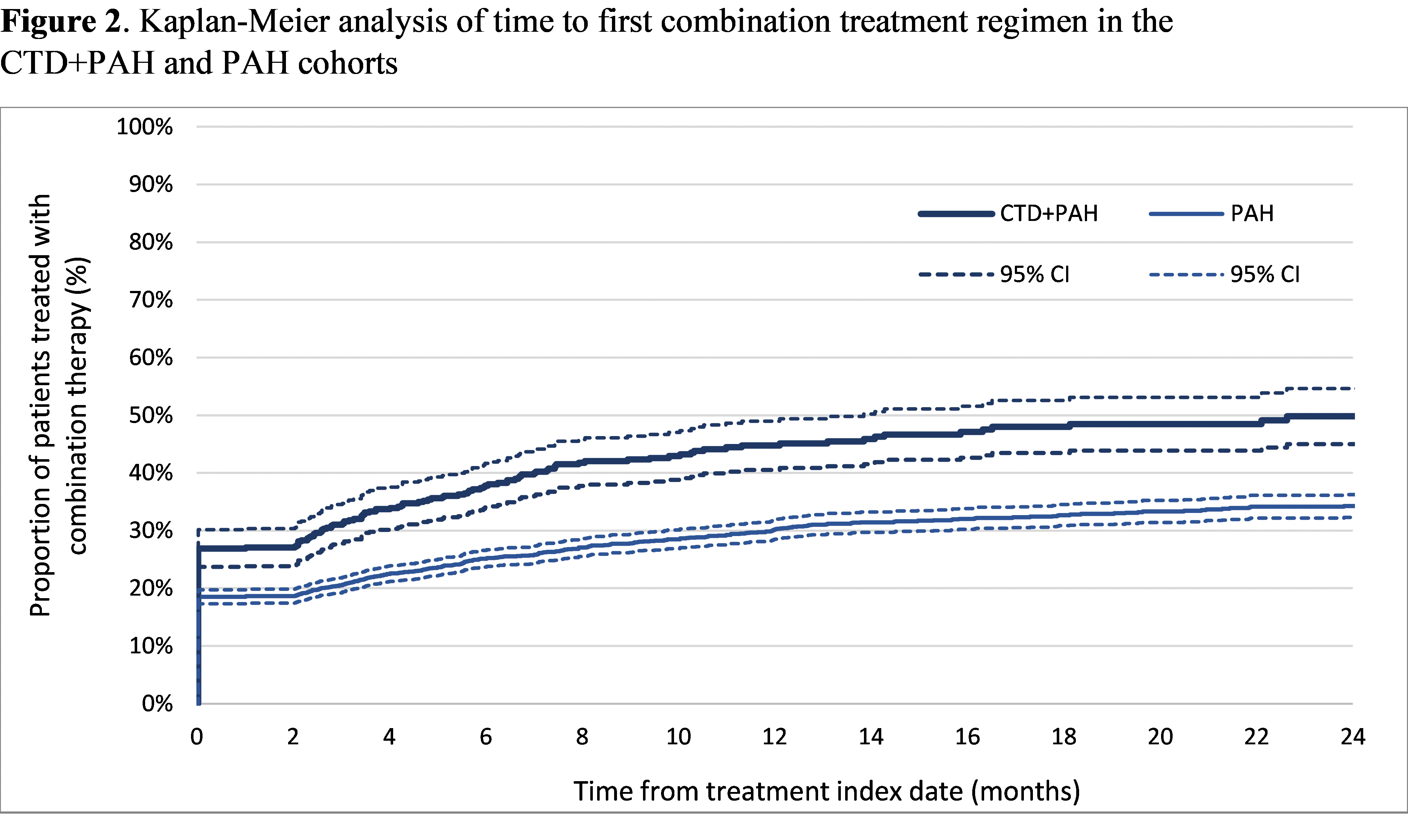 Abbreviations: CI: confidence interval; CTD: connective tissue disorder; PAH: pulmonary arterial hypertension.
Abbreviations: CI: confidence interval; CTD: connective tissue disorder; PAH: pulmonary arterial hypertension.
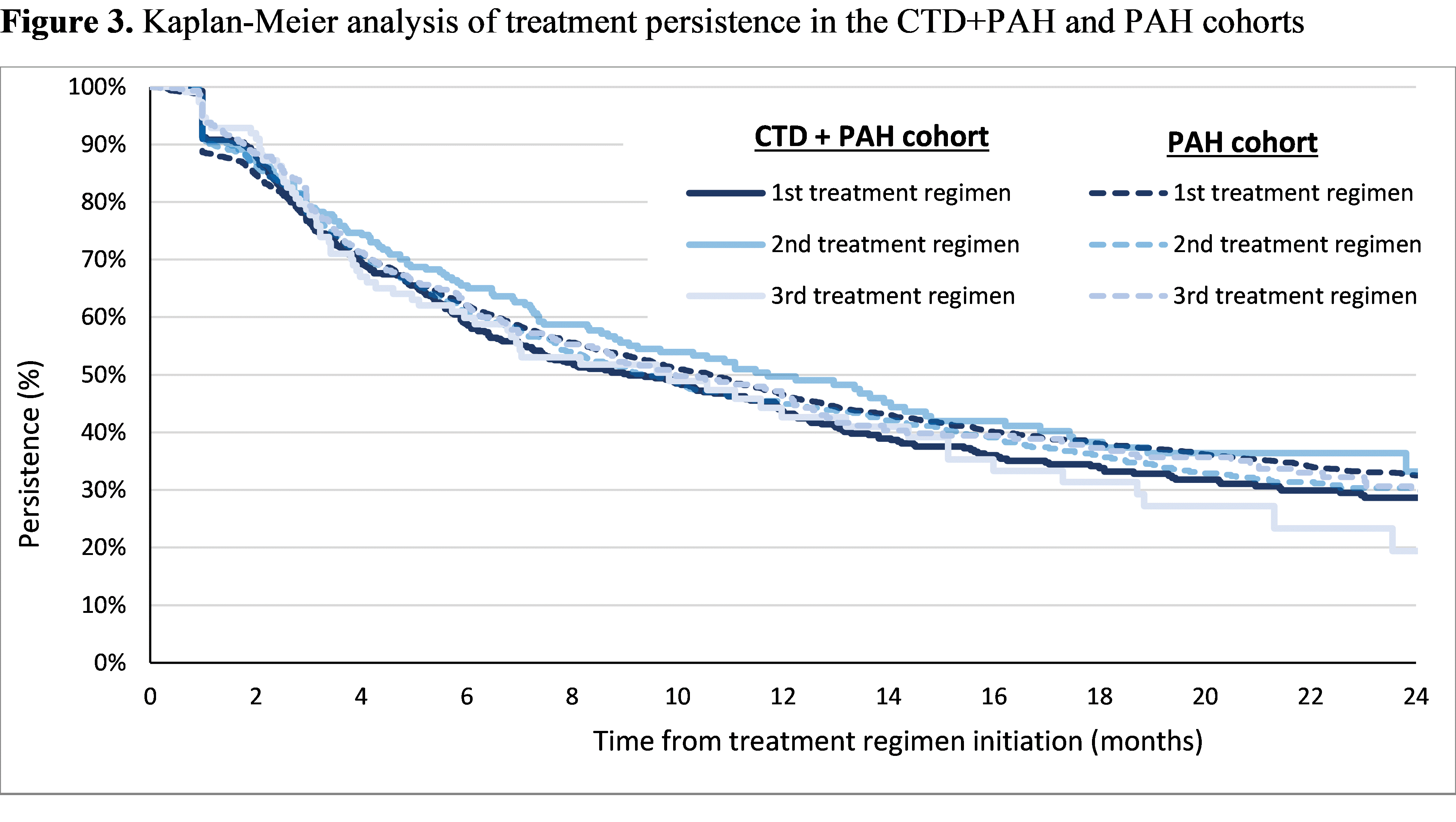 Abbreviations: CTD: connective tissue disorder; PAH: pulmonary arterial hypertension.
Abbreviations: CTD: connective tissue disorder; PAH: pulmonary arterial hypertension.
Note:
[1] Persistence was calculated as the time from the initiation of treatment regimen to the first PAH-related treatment change observed (i.e., treatment discontinuation, treatment switch, treatment augmentation, or treatment drop), whichever occurred first. Patients who remained on treatment were censored at the end of the patient follow-up.
Disclosures: Y. Tsang, Janssen; M. Gauthier-Loiselle, Janssen; V. Funtanilla, None; H. Germack, Janssen Scientific Affairs, LLC; A. Manceur, Janssen; S. Liu, Janssen; M. Cloutier, Janssen; P. Lefebvre, Janssen Scientific Affairs, LLC; S. Panjabi, Janssen.
Background/Purpose: Connective tissue disorders (CTDs) are the most frequent diseases associated with pulmonary arterial hypertension (PAH), accounting for 11% – 28% of PAH cases. A better understanding of real-world treatment patterns could help identify potential areas for improvement in the management of CTD-related PAH; thus, this study assessed PAH treatment patterns in this population in the US.
Methods: Adult patients initiated on a PAH treatment (index date: 1st initiation date) were identified from Optum Clinformatics® Data Mart Databases (10/01/2015-09/30/2021). Patients had ≥1 inpatient or ≥2 outpatient PAH diagnosis claims and ≥6 months of continuous insurance eligibility without PAH treatment before the index date (i.e., baseline period). Eligible patients were categorized into mutually exclusive cohorts: (1) CTD+PAH: had ≥1 inpatient or ≥2 outpatient CTD diagnosis claims, with ≥1 claim before/on the index date; or (2) PAH: no CTD diagnosis at any time during baseline or follow-up. Treatment patterns were assessed from the index date to the earliest of death, end of continuous insurance eligibility, or end of data availability. A treatment regimen was defined as all PAH agents observed within 60 days of the 1st agent; a ≥90-day gap with no PAH agents indicated treatment discontinuation. Kaplan-Meier (KM) analysis of persistence (time to 1st treatment change) and time to combination therapy was performed.
Results: Among 728 patients in the CTD+PAH cohort and 4,023 patients in the PAH cohort, average follow-up was 18.8 and 19.6 months, respectively. Mean age was 64.2 and 69.1 years in the CTD+PAH and PAH cohorts, with 85.7% and 60.8% female, respectively. For both cohorts, the most common 1st treatment regimens observed were sildenafil (CTD+PAH: 38.7%; PAH: 51.5%), tadalafil (10.0%; 9.4%), and macitentan (8.1%; 5.4%) in monotherapy. Sildenafil, tadalafil, and macitentan were also the most frequent agents included in any of the first 3 treatment regimens (Figure 1). Combination therapy was more frequent in the CTD+PAH cohort than in the PAH cohort (any regimen: 40.9% vs 27.2%; 1st treatment regimen: 26.9% vs 18.5%; 2nd: 52.8% vs 42.0%; 3rd: 55.2% vs 48.5%; Figure 1). KM analysis showed that patients in the CTD+PAH cohort were more likely to be initiated on combination therapy at any time post-index (Figure 2). The vast majority of patients had cardiologist, pulmonologist, and/or rheumatologist visits, but time to combination therapy was similar regardless of the type of specialist visits. Treatment persistence was similar across cohorts and first 3 treatment regimens, with persistence rates ranging from 42.6% to 49.7% at 12 months (Figure 3).
Conclusion: Compared to PAH patients, CTD+PAH patients were younger and more likely to be female. Treatment patterns were generally similar between the CTD+PAH and PAH cohorts, though combination therapy was more frequent in the CTD+PAH cohort. Both cohorts may benefit from broader use of all available PAH treatment classes, including combination therapy to target the nitric oxide, endothelin, and prostacyclin pathways. Our findings also highlight the need to understand low persistence rates with PAH therapies.
.jpg) Abbreviations: CTD: connective tissue disorder; ERA: endothelin receptor antagonist; PAH: pulmonary arterial hypertension; PDE5i: phosphodiesterase type 5 inhibitor; PPA, prostacyclin pathway agent; SGC: soluble guacylate cyclase inhibitor.
Abbreviations: CTD: connective tissue disorder; ERA: endothelin receptor antagonist; PAH: pulmonary arterial hypertension; PDE5i: phosphodiesterase type 5 inhibitor; PPA, prostacyclin pathway agent; SGC: soluble guacylate cyclase inhibitor.Note:
[1] All agents received are listed regardless of whether they were used as monotherapy or combination therapy.
 Abbreviations: CI: confidence interval; CTD: connective tissue disorder; PAH: pulmonary arterial hypertension.
Abbreviations: CI: confidence interval; CTD: connective tissue disorder; PAH: pulmonary arterial hypertension. Abbreviations: CTD: connective tissue disorder; PAH: pulmonary arterial hypertension.
Abbreviations: CTD: connective tissue disorder; PAH: pulmonary arterial hypertension.Note:
[1] Persistence was calculated as the time from the initiation of treatment regimen to the first PAH-related treatment change observed (i.e., treatment discontinuation, treatment switch, treatment augmentation, or treatment drop), whichever occurred first. Patients who remained on treatment were censored at the end of the patient follow-up.
Disclosures: Y. Tsang, Janssen; M. Gauthier-Loiselle, Janssen; V. Funtanilla, None; H. Germack, Janssen Scientific Affairs, LLC; A. Manceur, Janssen; S. Liu, Janssen; M. Cloutier, Janssen; P. Lefebvre, Janssen Scientific Affairs, LLC; S. Panjabi, Janssen.

