Back
Abstract Session
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: Abstracts: Systemic Sclerosis and Related Disorders – Clinical I: Trials and Therapeutics (0518–0523)
0518: Changes in Skin Fibroblast Polarization Gene Expression Herald Clinical Improvement in Early, Diffuse Cutaneous Systemic Sclerosis
Saturday, November 12, 2022
3:00 PM – 3:10 PM Eastern Time
Location: Room 121
- KL
Kimberly S. Lakin, MD, MS
Hospital for Special Surgery
New York, NY, United States
Presenting Author(s)
Kimberly Showalter Lakin1, Deanna Jannat-Khah, DrPH, MSPH1, Yajas Shah2, Olivier Elemento2, Aliza Bloostein1, Jessica Gordon1, Michael Whitfield3, Barbara White4, Dana Orange5 and Robert Spiera1, 1Hospital for Special Surgery, New York, NY, 2Weill Cornell Medicine, New York, NY, 3Dartmouth Geisel School of Medicine, Department of Biomedical Data Science, Lebanon, NH, 4Corbus Pharmaceuticals, Pleasanton, CA, 5The Rockefeller University, New York, NY
Background/Purpose: We previously identified a group of genes associated with high alpha-smooth muscle actin (aSMA) and low CD34 fibroblast staining in lesional systemic sclerosis (SSc) skin that decreased with clinical improvement in patients treated with belimumab or nilotinib. The purpose of this study is to evaluate this aSMA/CD34 (fibroblast) polarization gene expression signature as a biomarker of clinical improvement in a larger validation cohort of individuals with early, diffuse cutaneous (dc) SSc.
Methods: Skin biopsies were analyzed from 81 patients with dcSSc enrolled in the Phase III RESOLVE-1 clinical trial of lenabasum in SSc. The trial did not demonstrate lenabasum to be superior to placebo, so for the purposes of this analysis we did not stratify by treatment assignment. Clinical data were collected including patient demographics, SSc autoantibody profile, disease duration, modified Rodnan skin score (MRSS), and 52-week Combined Response Index in Systemic Sclerosis (CRISS). 52-week "clinical improvement" was defined as CRISS ≥0.6. Expression of 46 previously identified aSMA/CD34 polarization genes, measured by Agilent microarray, was evaluated in each sample. Pearson correlation coefficients were calculated for change in aSMA/CD34 polarization gene expression (Log2(fold-change baseline vs. 52 weeks)) and 52-week (A) CRISS and (B) change in MRSS.
Results: Baseline characteristics of 81 patients are shown in Table 1. Among clinical improvers, 42 of the 46 aSMA/CD34 polarization genes significantly decreased between baseline and 52-weeks, while 0 of 46 genes changed significantly in clinical non-improvers (Fig. 1A). In the clinical improvers, the baseline samples clustered together, along with higher MRSS scores and higher aSMA/CD34 polarization gene expression, compared to 52-week samples (Fig. 1B). On the other hand, there was no clustering according to time (baseline vs. 52 weeks) in non-improver samples. Average expression of aSMA/CD34 polarization genes was plotted for improvers vs. non-improvers for baseline and 52-week samples. Average gene expression in improvers (vs. non-improvers) was similar at baseline but decreased at 52 weeks in improvers (Fig. 2A). LUM, SEPT11, SILP2 and CFI were most strongly correlated with 52-week CRISS, (Fig. 2B), and CILP2, TNFSF4, SYNDIG1 and INHBA were most strongly correlated with MRSS change (Fig. 2C).
Conclusion: The gene expression signature of aSMA/CD34 polarization decreases significantly from baseline to 52 weeks among clinical improvers but is unchanged in those who do not improve. These results validate previous findings that aSMA/CD34 polarization gene expression signatures decrease in patients with clinical improvement and suggest fibroblasts can recover, potentially by repopulating or differentiating, in the improving skin of patients with SSc.
.jpg)
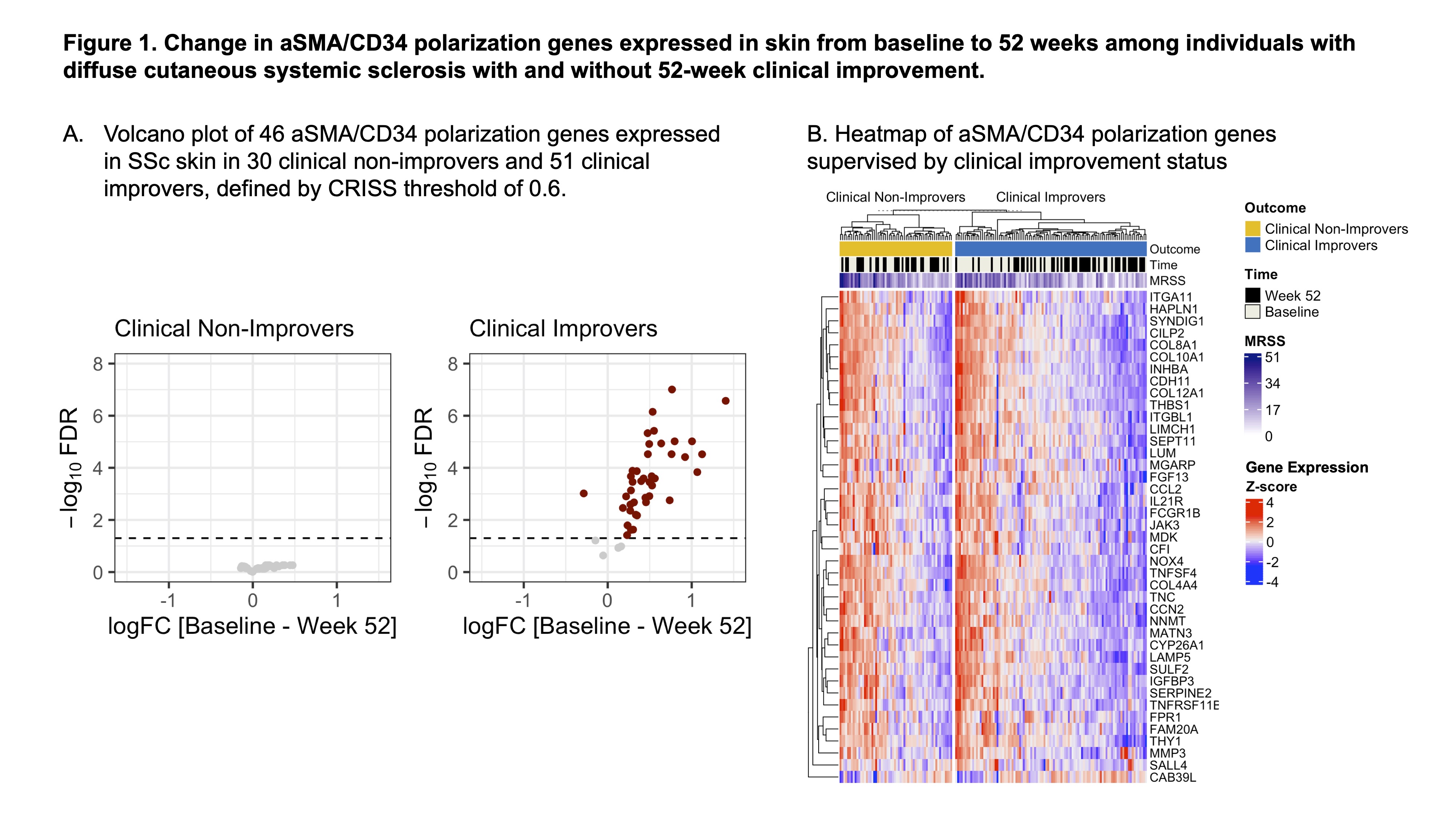 Figure 1. Change in aSMA/CD34 polarization genes expressed in skin from baseline to 52 weeks among individuals with diffuse cutaneous systemic sclerosis with and without 52-week clinical improvement. (A) Volcano plot of 46 aSMA/CD34 polarization genes expressed in SSc skin in 30 clinical non-improvers and 51 clinical improvers, defined by CRISS threshold of 0.6. (B) Heatmap of aSMA/CD34 polarization genes supervised by clinical improvement status.
Figure 1. Change in aSMA/CD34 polarization genes expressed in skin from baseline to 52 weeks among individuals with diffuse cutaneous systemic sclerosis with and without 52-week clinical improvement. (A) Volcano plot of 46 aSMA/CD34 polarization genes expressed in SSc skin in 30 clinical non-improvers and 51 clinical improvers, defined by CRISS threshold of 0.6. (B) Heatmap of aSMA/CD34 polarization genes supervised by clinical improvement status.
.jpg) Figure 2. Change in aSMA/CD34 polarization gene expression in skin compared to clinical improvement. (A) Average expression of aSMA/CD34 polarization genes at baseline and 52 weeks by clinical improvement status. Red line indicates equal average gene expression between clinical improvers and non-improvers. (B) Heatmap demonstrates Pearson correlation coefficients for Log2(fold-change baseline versus 52 weeks) of 46 aSMA/CD34 polarization genes with (A) CRISS and (B) total MRSS change, measured as baseline minus 52 weeks (i.e., positive MRSS change indicates points of “improvement” in total skin score at 52 weeks).
Figure 2. Change in aSMA/CD34 polarization gene expression in skin compared to clinical improvement. (A) Average expression of aSMA/CD34 polarization genes at baseline and 52 weeks by clinical improvement status. Red line indicates equal average gene expression between clinical improvers and non-improvers. (B) Heatmap demonstrates Pearson correlation coefficients for Log2(fold-change baseline versus 52 weeks) of 46 aSMA/CD34 polarization genes with (A) CRISS and (B) total MRSS change, measured as baseline minus 52 weeks (i.e., positive MRSS change indicates points of “improvement” in total skin score at 52 weeks).
Disclosures: K. Showalter Lakin, None; D. Jannat-Khah, DrPH, MSPH, Cytodyn, AstraZeneca, Walgreens; Y. Shah, None; O. Elemento, None; A. Bloostein, None; J. Gordon, None; M. Whitfield, Corbus Pharmaceuticals, Celdara Medical LLC, Bristol-Myers Squibb(BMS); B. White, Corbus Pharmaceuticals, Inc.; D. Orange, None; R. Spiera, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche, Vera.
Background/Purpose: We previously identified a group of genes associated with high alpha-smooth muscle actin (aSMA) and low CD34 fibroblast staining in lesional systemic sclerosis (SSc) skin that decreased with clinical improvement in patients treated with belimumab or nilotinib. The purpose of this study is to evaluate this aSMA/CD34 (fibroblast) polarization gene expression signature as a biomarker of clinical improvement in a larger validation cohort of individuals with early, diffuse cutaneous (dc) SSc.
Methods: Skin biopsies were analyzed from 81 patients with dcSSc enrolled in the Phase III RESOLVE-1 clinical trial of lenabasum in SSc. The trial did not demonstrate lenabasum to be superior to placebo, so for the purposes of this analysis we did not stratify by treatment assignment. Clinical data were collected including patient demographics, SSc autoantibody profile, disease duration, modified Rodnan skin score (MRSS), and 52-week Combined Response Index in Systemic Sclerosis (CRISS). 52-week "clinical improvement" was defined as CRISS ≥0.6. Expression of 46 previously identified aSMA/CD34 polarization genes, measured by Agilent microarray, was evaluated in each sample. Pearson correlation coefficients were calculated for change in aSMA/CD34 polarization gene expression (Log2(fold-change baseline vs. 52 weeks)) and 52-week (A) CRISS and (B) change in MRSS.
Results: Baseline characteristics of 81 patients are shown in Table 1. Among clinical improvers, 42 of the 46 aSMA/CD34 polarization genes significantly decreased between baseline and 52-weeks, while 0 of 46 genes changed significantly in clinical non-improvers (Fig. 1A). In the clinical improvers, the baseline samples clustered together, along with higher MRSS scores and higher aSMA/CD34 polarization gene expression, compared to 52-week samples (Fig. 1B). On the other hand, there was no clustering according to time (baseline vs. 52 weeks) in non-improver samples. Average expression of aSMA/CD34 polarization genes was plotted for improvers vs. non-improvers for baseline and 52-week samples. Average gene expression in improvers (vs. non-improvers) was similar at baseline but decreased at 52 weeks in improvers (Fig. 2A). LUM, SEPT11, SILP2 and CFI were most strongly correlated with 52-week CRISS, (Fig. 2B), and CILP2, TNFSF4, SYNDIG1 and INHBA were most strongly correlated with MRSS change (Fig. 2C).
Conclusion: The gene expression signature of aSMA/CD34 polarization decreases significantly from baseline to 52 weeks among clinical improvers but is unchanged in those who do not improve. These results validate previous findings that aSMA/CD34 polarization gene expression signatures decrease in patients with clinical improvement and suggest fibroblasts can recover, potentially by repopulating or differentiating, in the improving skin of patients with SSc.
.jpg)
 Figure 1. Change in aSMA/CD34 polarization genes expressed in skin from baseline to 52 weeks among individuals with diffuse cutaneous systemic sclerosis with and without 52-week clinical improvement. (A) Volcano plot of 46 aSMA/CD34 polarization genes expressed in SSc skin in 30 clinical non-improvers and 51 clinical improvers, defined by CRISS threshold of 0.6. (B) Heatmap of aSMA/CD34 polarization genes supervised by clinical improvement status.
Figure 1. Change in aSMA/CD34 polarization genes expressed in skin from baseline to 52 weeks among individuals with diffuse cutaneous systemic sclerosis with and without 52-week clinical improvement. (A) Volcano plot of 46 aSMA/CD34 polarization genes expressed in SSc skin in 30 clinical non-improvers and 51 clinical improvers, defined by CRISS threshold of 0.6. (B) Heatmap of aSMA/CD34 polarization genes supervised by clinical improvement status..jpg) Figure 2. Change in aSMA/CD34 polarization gene expression in skin compared to clinical improvement. (A) Average expression of aSMA/CD34 polarization genes at baseline and 52 weeks by clinical improvement status. Red line indicates equal average gene expression between clinical improvers and non-improvers. (B) Heatmap demonstrates Pearson correlation coefficients for Log2(fold-change baseline versus 52 weeks) of 46 aSMA/CD34 polarization genes with (A) CRISS and (B) total MRSS change, measured as baseline minus 52 weeks (i.e., positive MRSS change indicates points of “improvement” in total skin score at 52 weeks).
Figure 2. Change in aSMA/CD34 polarization gene expression in skin compared to clinical improvement. (A) Average expression of aSMA/CD34 polarization genes at baseline and 52 weeks by clinical improvement status. Red line indicates equal average gene expression between clinical improvers and non-improvers. (B) Heatmap demonstrates Pearson correlation coefficients for Log2(fold-change baseline versus 52 weeks) of 46 aSMA/CD34 polarization genes with (A) CRISS and (B) total MRSS change, measured as baseline minus 52 weeks (i.e., positive MRSS change indicates points of “improvement” in total skin score at 52 weeks).Disclosures: K. Showalter Lakin, None; D. Jannat-Khah, DrPH, MSPH, Cytodyn, AstraZeneca, Walgreens; Y. Shah, None; O. Elemento, None; A. Bloostein, None; J. Gordon, None; M. Whitfield, Corbus Pharmaceuticals, Celdara Medical LLC, Bristol-Myers Squibb(BMS); B. White, Corbus Pharmaceuticals, Inc.; D. Orange, None; R. Spiera, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche, Vera.

