Back
Poster Session D
Session: (2108–2153) Spondyloarthritis Including PsA – Treatment Poster III: PsA
2115: Early Skin and Early Enthesitis Responses in Psoriatic Arthritis Patients Treated with Guselkumab Associate with Long-term Response: Post Hoc Analysis Through 2 Years of a Phase 3 Study
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.jpg)
Laura C. Coates, MD, PhD
University of Oxford
Oxford, United Kingdom
Abstract Poster Presenter(s)
Laura Coates1, Julio Ramírez2, Dennis McGonagle3, Sibel Aydin4, Miriam Zimmermann5, Francois Nantel6, May Shawi7, Emmanouil Rampakakis8, Peter Nash9 and Philip J Mease10, 1Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK, Oxford, England, United Kingdom, 2Arthritis Unit, Rheumatology Department, Hospital Clinic, Barcelona, Spain, 3University of Leeds, Leeds, UK, Leeds, United Kingdom, 4University of Ottawa, Ottawa Hospital Research Institute, Ottawa, ON, Canada, 5Immunology, Janssen Scientific Affairs, LLC, Zug, Switzerland, 6Nantel MedSci Consult, Montréal, QC, Canada, 7Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, Horsham, PA, 8McGill University, Department of Pediatrics and JSS Medical Research, Montréal, QC, Canada, 9School of Medicine, Griffith University, Sunshine Coast, Australia, 10Swedish Medical Center/Providence St. Joseph Health, Seattle, WA
Background/Purpose: Guselkumab (GUS), an IL-23p19 inhibitor, has demonstrated efficacy in PsA across key Group for Research and Assessment of Psoriasis (PsO) and Psoriatic Arthritis (GRAPPA)-recommended domains1,2. Skin disease and enthesitis have been identified as disease manifestations with earlier response times than others3. In this analysis we: (a) Determined whether early skin and/or entheseal response predicts future response in other PsA domains; (b) Evaluated the trajectory for achieving skin/entheseal responses by 52 weeks (W) in patients (pts) without early responses.
Methods: Pts in the DISCOVER-1 and DISCOVER-2 (D1/2) studies were adults with active PsA despite standard therapies. D1 pts had ≥3 swollen and ≥3 tender joints (SJC/TJC) and C-reactive protein (CRP) ≥0.3 mg/dL; D2 pts had SJC ≥5, TJC ≥5, and CRP ≥0.6 mg/dL. 31% of D1 pts received 1-2 prior TNF inhibitors; D2 pts were biologic-naïve. Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo. These post hoc analyses included only pooled GUS Q4W and Q8W pts (N=748). Early skin response was defined as PsO Area and Severity Index (PASI) score ≤1 at W16 or skin visual analogue scale (VAS) ≤15mm at W8 among pts with a baseline (BL) PASI score >1 and skin VAS >15mm (first assessment time for both); early entheseal response as Leeds Enthesitis Index (LEI) score ≤1 at W8; and categories of early response defined as skin VAS ≤15mm only vs LEI score ≤1 only vs combined skin VAS ≤15mm & LEI score ≤1 vs none at W8. Potential responses at W24 & W52 included achievement of minimal disease activity (MDA), Disease Activity in PsA (DAPSA) low disease activity (LDA) or remission, DAPSA50, and enthesitis/dactylitis resolution. Associations between early skin/entheseal response and W24/W52 response were assessed with crosstabulations and logistic regression.
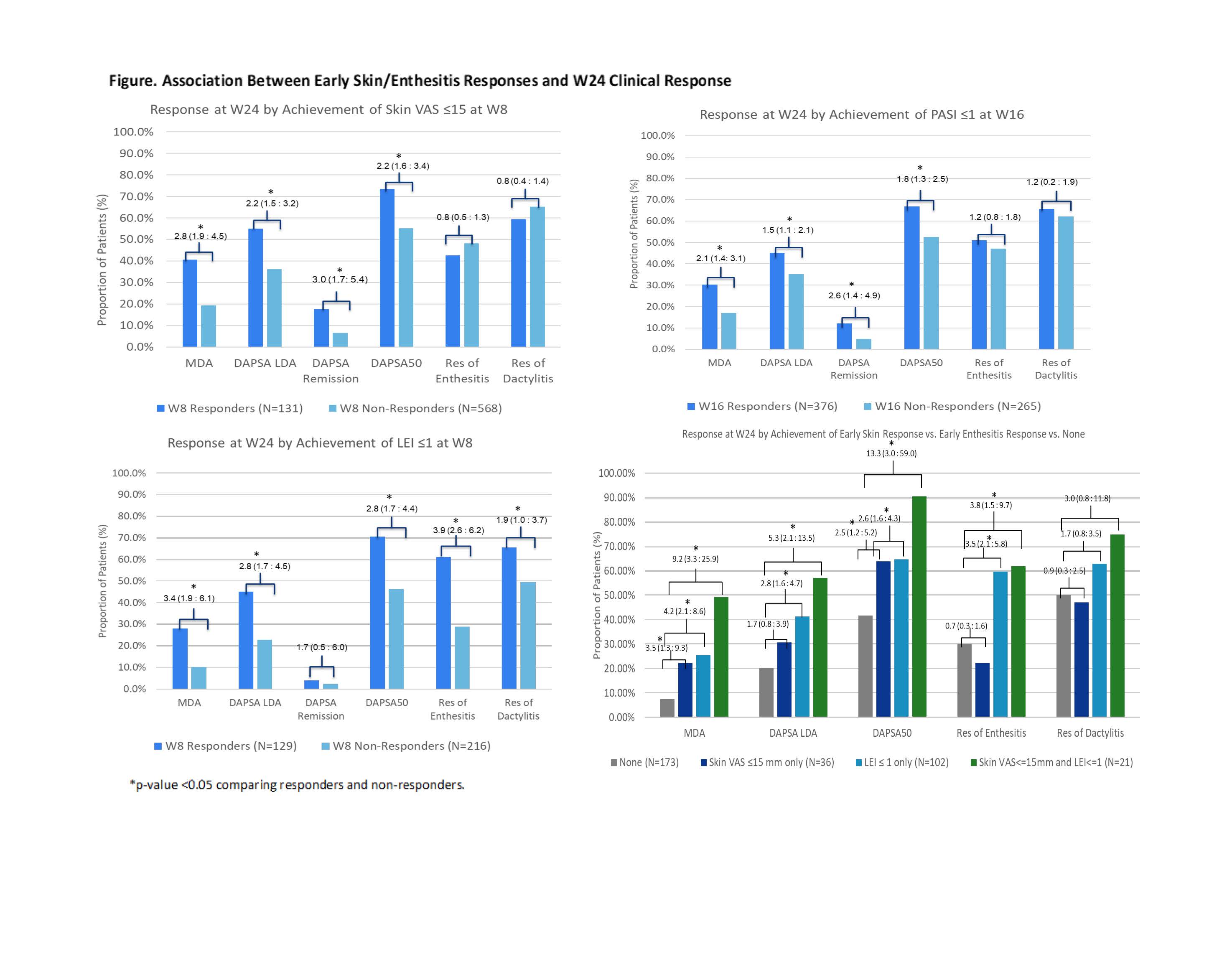
Results: Early skin response associated with greater odds of achieving W24 MDA, DAPSA LDA, DAPSA remission, and DAPSA50, but not enthesitis or dactylitis resolution (Figure). Early entheseal response associated with greater odds of achieving all W24 outcomes, including resolution of enthesitis or dactylitis, with the exception of DAPSA remission; DAPSA remission was achieved by a greater proportion of early responders, though the association was significant only at W52. In pts with both BL PsO and enthesitis, early responders in both domains were even more likely to subsequently demonstrate MDA, DAPSA LDA, DAPSA50, DAPSA remission only at W52, and dactylitis resolution than pts with individual responses. Among pts who did not achieve early responses, approximately half did so by W52.
Conclusion: Early skin and entheseal responses predicted long-term clinical response, including disease remission. A synergistic effect was observed, in which pts with BL PsO and enthesitis exhibiting early response in both domains were more likely to achieve later clinical response. These results highlight the importance of early response in these two domains on the trajectory of long-term pt outcome.
References:
1. Deodhar A et al. Lancet. 2020;395(10230) :1115-25
2. Mease PJ et al. Lancet. 2020;395(10230):1125-36
3. Coates LC et al. A893. EULAR 2022, Denmark
Disclosures: L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK); J. Ramírez, AbbVie/Abbott, Amgen, Eli Lilly, Janssen, Novartis, UCB; D. McGonagle, Abbvie, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, UCB, Lilly, MSD, Roche, Sobi; S. Aydin, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, UCB; M. Zimmermann, Janssen Scientific Affairs, LLC, Johnson & Johnson; F. Nantel, Janssen, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; E. Rampakakis, Janssen, JSS Medical Research; P. Nash, AbbVie, Eli Lilly, Janssen, Gilead, Bristol-Myers Squibb (BMS), Celgene; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.
Background/Purpose: Guselkumab (GUS), an IL-23p19 inhibitor, has demonstrated efficacy in PsA across key Group for Research and Assessment of Psoriasis (PsO) and Psoriatic Arthritis (GRAPPA)-recommended domains1,2. Skin disease and enthesitis have been identified as disease manifestations with earlier response times than others3. In this analysis we: (a) Determined whether early skin and/or entheseal response predicts future response in other PsA domains; (b) Evaluated the trajectory for achieving skin/entheseal responses by 52 weeks (W) in patients (pts) without early responses.
Methods: Pts in the DISCOVER-1 and DISCOVER-2 (D1/2) studies were adults with active PsA despite standard therapies. D1 pts had ≥3 swollen and ≥3 tender joints (SJC/TJC) and C-reactive protein (CRP) ≥0.3 mg/dL; D2 pts had SJC ≥5, TJC ≥5, and CRP ≥0.6 mg/dL. 31% of D1 pts received 1-2 prior TNF inhibitors; D2 pts were biologic-naïve. Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo. These post hoc analyses included only pooled GUS Q4W and Q8W pts (N=748). Early skin response was defined as PsO Area and Severity Index (PASI) score ≤1 at W16 or skin visual analogue scale (VAS) ≤15mm at W8 among pts with a baseline (BL) PASI score >1 and skin VAS >15mm (first assessment time for both); early entheseal response as Leeds Enthesitis Index (LEI) score ≤1 at W8; and categories of early response defined as skin VAS ≤15mm only vs LEI score ≤1 only vs combined skin VAS ≤15mm & LEI score ≤1 vs none at W8. Potential responses at W24 & W52 included achievement of minimal disease activity (MDA), Disease Activity in PsA (DAPSA) low disease activity (LDA) or remission, DAPSA50, and enthesitis/dactylitis resolution. Associations between early skin/entheseal response and W24/W52 response were assessed with crosstabulations and logistic regression.
Results: Early skin response associated with greater odds of achieving W24 MDA, DAPSA LDA, DAPSA remission, and DAPSA50, but not enthesitis or dactylitis resolution (Figure). Early entheseal response associated with greater odds of achieving all W24 outcomes, including resolution of enthesitis or dactylitis, with the exception of DAPSA remission; DAPSA remission was achieved by a greater proportion of early responders, though the association was significant only at W52. In pts with both BL PsO and enthesitis, early responders in both domains were even more likely to subsequently demonstrate MDA, DAPSA LDA, DAPSA50, DAPSA remission only at W52, and dactylitis resolution than pts with individual responses. Among pts who did not achieve early responses, approximately half did so by W52.
Conclusion: Early skin and entheseal responses predicted long-term clinical response, including disease remission. A synergistic effect was observed, in which pts with BL PsO and enthesitis exhibiting early response in both domains were more likely to achieve later clinical response. These results highlight the importance of early response in these two domains on the trajectory of long-term pt outcome.
References:
1. Deodhar A et al. Lancet. 2020;395(10230) :1115-25
2. Mease PJ et al. Lancet. 2020;395(10230):1125-36
3. Coates LC et al. A893. EULAR 2022, Denmark

Disclosures: L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK); J. Ramírez, AbbVie/Abbott, Amgen, Eli Lilly, Janssen, Novartis, UCB; D. McGonagle, Abbvie, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, UCB, Lilly, MSD, Roche, Sobi; S. Aydin, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, UCB; M. Zimmermann, Janssen Scientific Affairs, LLC, Johnson & Johnson; F. Nantel, Janssen, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; E. Rampakakis, Janssen, JSS Medical Research; P. Nash, AbbVie, Eli Lilly, Janssen, Gilead, Bristol-Myers Squibb (BMS), Celgene; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.

