Back
Poster Session D
Session: (2108–2153) Spondyloarthritis Including PsA – Treatment Poster III: PsA
2143: Effect of Upadacitinib and Adalimumab on Residual Pain Among Patients with Psoriatic Arthritis Whose Inflammation Was Attenuated After Three and Six Months of Treatment
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LB
Louis Bessette, MD, MSc
Centre de l'Ostéoporose et de Rhumatologie de Québec
Quebec, QC, Canada
Abstract Poster Presenter(s)
Louis Bessette1, Georg Pongratz2, Luca Navarini3, Rodrigo Garcia Salinas4, Tianming Gao5, Marie-Claude Laliberté6, Ralph Lippe7 and Philip J Mease8, 1Centre de l'Ostoporose et de Rhumatologie de Québec, Québec, QC, Canada, 2Asklepios Clinic Bad Abbach, University of Regensburg, Regensburg, Germany, 3Rheumatology, Immunology, and Clinical Medicine, Department of Medicine, Campus Bio-Medico University of Rome, Rome, Italy, 4Hospital Italiano de La Plata, La Plata, Argentina, 5AbbVie, Inc., North Chicago, IL, 6AbbVie, Québec, QC, Canada, 7AbbVie, Inc, Wiesbaden, Germany, 8Swedish Medical Center/Providence St. Joseph Health, Seattle, WA
Background/Purpose: Managing pain, a predominant symptom of psoriatic arthritis (PsA), is a priority for patients and healthcare providers. Upadacitinib (UPA), a Janus kinase (JAK) inhibitor, was effective vs placebo (PBO) in the SELECT-PsA 1 study. The objective of this post-hoc analysis was to evaluate the efficacy of UPA, adalimumab (ADA), and PBO on residual pain in patients with PsA who had attenuation of inflammation.
Methods: The SELECT-PsA 1 study enrolled adults with active PsA with prior inadequate response or intolerance to ≥1 non-biologic DMARD. Randomization arms included UPA 15 mg once daily (QD), ADA 40 mg every other week, and PBO. Subgroup assessment was conducted between patients with attenuation of inflammation vs remaining inflammation at week 12 and week 24. Attenuation of inflammation was defined as swollen joint count based on 66 joints (SJC66) of 0 and CRP levels < 6 mg/L. Mean change from baseline in Patient's Global Assessment (PGA) of pain at week 12 and week 24 as well as ≥30%, ≥50%, or ≥70% reduction from baseline to week 12 and week 24 in PGA of pain were assessed. For continuous endpoints, analysis of covariance (ANCOVA) was performed at week 12 and 24 separately. The ANCOVA models included treatment and current DMARD use (yes/no) as fixed factors, and baseline value as covariate. For binary endpoints, as observed approach was used, and 95% CI for response rate and response rate difference was based on normal approximation. Patients who received rescue therapy after week 16 were excluded from the week 24 analysis.
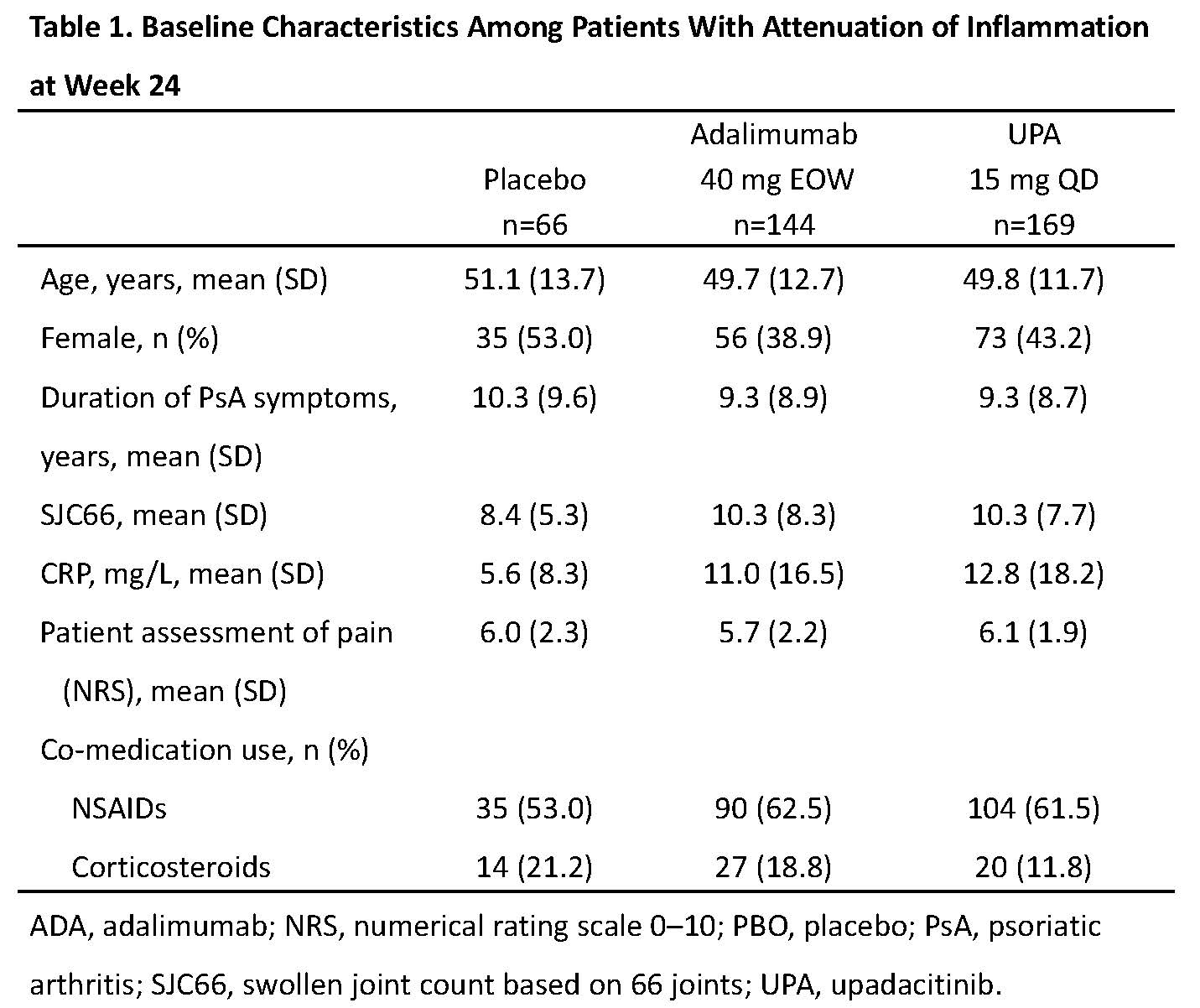
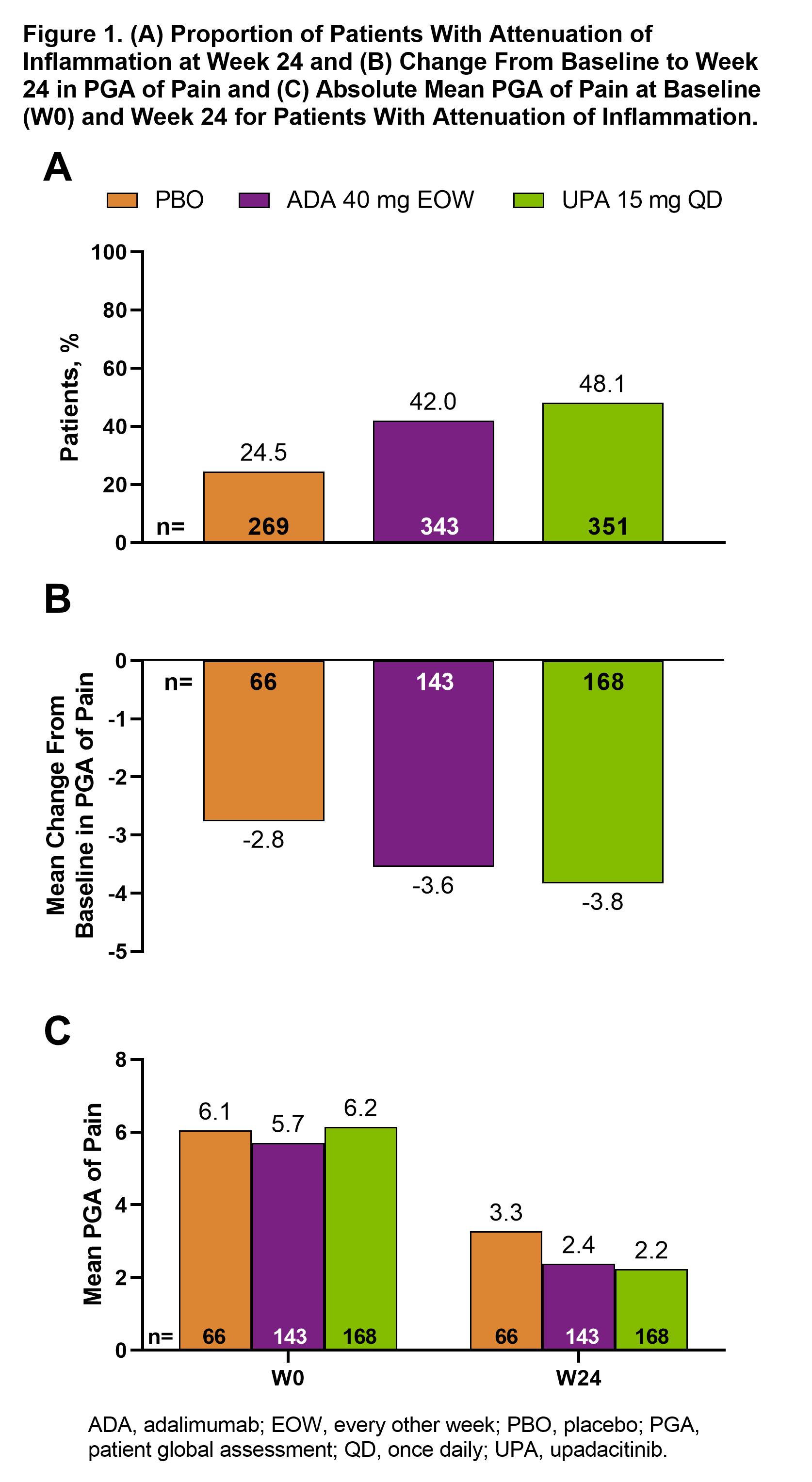
Results: Attenuation of inflammation at week 24 was reached by 169 (48.1%), 144 (42.0%), and 66 (24.5%) patients receiving UPA, ADA, and PBO, respectively. Some differences in baseline characteristics were observed between treatment groups in the attenuation of inflammation group, especially in CRP levels (Table 1). Among these patients, mean (95% CI) PGA of pain improved more for UPA (–3.8 [–4.2, –3.5]) and ADA (–3.6 [–3.9, –3.2]) vs PBO (–2.8 [–3.3, –2.3]) at week 24 (Figure 1A). Absolute mean values for PGA dropped from 6.2 at baseline to 2.2 at week 24 with UPA (Figure 1B). For ≥30%, ≥50%, or ≥70% reduction in PGA of pain from baseline, response rates with UPA and ADA were higher than PBO at weeks 12 and 24 (Figure 2). Among patients with remaining inflammation, a similar trend was observed across endpoints at weeks 12 and 24.
Conclusion: After 24 weeks, nearly half of the patients treated with UPA had attenuation of inflammation. In these patients, mean PGA of pain dropped from 6.2 at baseline to 2.2 at week 24, close to the ≤2.0 threshold representing when satisfaction with health is not negatively affected by pain.1,2 Both UPA and ADA showed a higher response rate vs PBO. These results suggest that both UPA and ADA are effective in reducing residual pain in PsA patients over 6 months.
References
1. Wolfe F and Michaud K. J Rheumatol. 2007;34(8):1674-83.
2. Wells GA, et al. J Rheumatol. 2005;32(10):2016-24.
.jpg)
Disclosures: L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; G. Pongratz, Lilly, Sanofi, Boehringer, AbbVie, Galapagos; L. Navarini, BMS, Janssen-Cilag, Novartis, MSD, Celegne, AbbVie, Pfizer, Italfarmaco, Roche, Eli Lilly, Sanofi-Genzyme, UCB, Philogen, SOBI, Galapagos; R. Garcia Salinas, Eli Lilly, Janssen, Novartis, GlaxoSmithKlein(GSK), Genentech, BMS, Merck, UCB, Pfizer; T. Gao, AbbVie; M. Laliberté, AbbVie Inc.; R. Lippe, AbbVie; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.
Background/Purpose: Managing pain, a predominant symptom of psoriatic arthritis (PsA), is a priority for patients and healthcare providers. Upadacitinib (UPA), a Janus kinase (JAK) inhibitor, was effective vs placebo (PBO) in the SELECT-PsA 1 study. The objective of this post-hoc analysis was to evaluate the efficacy of UPA, adalimumab (ADA), and PBO on residual pain in patients with PsA who had attenuation of inflammation.
Methods: The SELECT-PsA 1 study enrolled adults with active PsA with prior inadequate response or intolerance to ≥1 non-biologic DMARD. Randomization arms included UPA 15 mg once daily (QD), ADA 40 mg every other week, and PBO. Subgroup assessment was conducted between patients with attenuation of inflammation vs remaining inflammation at week 12 and week 24. Attenuation of inflammation was defined as swollen joint count based on 66 joints (SJC66) of 0 and CRP levels < 6 mg/L. Mean change from baseline in Patient's Global Assessment (PGA) of pain at week 12 and week 24 as well as ≥30%, ≥50%, or ≥70% reduction from baseline to week 12 and week 24 in PGA of pain were assessed. For continuous endpoints, analysis of covariance (ANCOVA) was performed at week 12 and 24 separately. The ANCOVA models included treatment and current DMARD use (yes/no) as fixed factors, and baseline value as covariate. For binary endpoints, as observed approach was used, and 95% CI for response rate and response rate difference was based on normal approximation. Patients who received rescue therapy after week 16 were excluded from the week 24 analysis.
Results: Attenuation of inflammation at week 24 was reached by 169 (48.1%), 144 (42.0%), and 66 (24.5%) patients receiving UPA, ADA, and PBO, respectively. Some differences in baseline characteristics were observed between treatment groups in the attenuation of inflammation group, especially in CRP levels (Table 1). Among these patients, mean (95% CI) PGA of pain improved more for UPA (–3.8 [–4.2, –3.5]) and ADA (–3.6 [–3.9, –3.2]) vs PBO (–2.8 [–3.3, –2.3]) at week 24 (Figure 1A). Absolute mean values for PGA dropped from 6.2 at baseline to 2.2 at week 24 with UPA (Figure 1B). For ≥30%, ≥50%, or ≥70% reduction in PGA of pain from baseline, response rates with UPA and ADA were higher than PBO at weeks 12 and 24 (Figure 2). Among patients with remaining inflammation, a similar trend was observed across endpoints at weeks 12 and 24.
Conclusion: After 24 weeks, nearly half of the patients treated with UPA had attenuation of inflammation. In these patients, mean PGA of pain dropped from 6.2 at baseline to 2.2 at week 24, close to the ≤2.0 threshold representing when satisfaction with health is not negatively affected by pain.1,2 Both UPA and ADA showed a higher response rate vs PBO. These results suggest that both UPA and ADA are effective in reducing residual pain in PsA patients over 6 months.
References
1. Wolfe F and Michaud K. J Rheumatol. 2007;34(8):1674-83.
2. Wells GA, et al. J Rheumatol. 2005;32(10):2016-24.


.jpg)
Disclosures: L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; G. Pongratz, Lilly, Sanofi, Boehringer, AbbVie, Galapagos; L. Navarini, BMS, Janssen-Cilag, Novartis, MSD, Celegne, AbbVie, Pfizer, Italfarmaco, Roche, Eli Lilly, Sanofi-Genzyme, UCB, Philogen, SOBI, Galapagos; R. Garcia Salinas, Eli Lilly, Janssen, Novartis, GlaxoSmithKlein(GSK), Genentech, BMS, Merck, UCB, Pfizer; T. Gao, AbbVie; M. Laliberté, AbbVie Inc.; R. Lippe, AbbVie; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.

