Back
Abstract Session
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: Abstracts: Pediatric Rheumatology – Clinical I: Connective Tissue Disease (0512–0517)
0514: Renal Response Outcomes of North American Youth with Proliferative Lupus Nephritis Treated with the EuroLupus versus NIH Cyclophosphamide Dosing Regimen
Saturday, November 12, 2022
3:30 PM – 3:40 PM Eastern Time
Location: Room 126
- CW
Christine Wang, MD
University of Michigan
Ann Arbor, MI, United States
Presenting Author(s)
Christine Wang1, Rebecca Sadun2, Wenru Zhou3, Kristen Miller3, Claire Palmer3, Stacy P Ardoin4, Christine Bacha5, Emily Hause6, Joyce Hui-Yuen7, Nicole Ling8, Maria Pereira9, Meredith Riebschleger10, Kelly Rouster-Stevens11, Aliese Sarkissian12, Julia Shalen13, William Soulsby14, Marinka Twilt15, Eveline Wu16, Laura Lewandowski17, Scott Wenderfer18 and Jennifer Cooper19, 1Children's Hospital of Colorado/University of Colorado, Denver, CO, 2Duke University, Durham, NC, 3University of Colorado School of Medicine, Aurora, CO, 4Division of Rheumatology, Nationwide Children's Hospital and Department of Pediatrics, The Ohio State University, Columbus, OH, 5Nationwide Children's Hospital, Columbus, OH, 6University of Minnesota, Minneapolis, MN, 7Cohen Children’s Medical Center, Hofstra-Northwell School of Medicine, Feinstein Institutes for Medical Research, Queens, NY, 8UCSF, San Francisco, CA, 9Baylor College of Medicine, Houston, TX, 10University of Michigan, Ann Arbor, MI, 11Division of Pediatric Rheumatology, Department of Pediatrics, Emory University School of Medicine; Children’s Healthcare of Atlanta, Atlanta, GA, 12University of North Carolina at Chapel Hill, Chapel Hill, NC, 13Johns Hopkins University, Baltimore, MD, 14University of California, San Francisco, San Francisco, CA, 15Cumming School of Medicine, University of Calgary, Calgary, AB, Canada, 16University of North Carolina, Chapel Hill, NC, 17NIAMS, NIH, Bethesda, MD, 18British Columbia Children's Hospital, Vancouver, BC, Canada, 19Children's Hospital of Colorado/University of Colorado, Aurora, CO
Background/Purpose: There have been no published studies comparing renal outcomes of youth with proliferative lupus nephritis (LN) treated with the lower-dose EuroLupus (EL) cyclophosphamide (CYC) regimen vs. the NIH regimen. We aimed to evaluate the association between the CYC dosing regimen and renal response at 12 months.
Methods: A multisite retrospective cohort study was conducted at 11 North American pediatric centers using electronic medical record data. Patients 6 to 22 years of age with active proliferative LN treated with either the EL or NIH regimen from 7/1/2014 to 6/30/2021 were included. Data were obtained through 12 months after baseline (defined as start of most recent CYC course). The primary outcome was the proportion of patients with complete (CRR) or partial renal response (PRR) at 12 months. The secondary outcome was the proportion of patients with CRR at 12 months. CRR was defined as normal estimated glomerular filtration rate (eGFR > 90 mL/min/1.72 m2) and urine protein-to-creatinine ratio (UPCR) < 0.2. PRR was defined as ≥ 50% improvement in eGFR and UPCR if abnormal at baseline and UPCR ≤ 1. Urine sediment was excluded from response definitions due to inability to exclude concurrent menses (hematuria), infection (pyuria) and missing values (casts). Baseline characteristics were compared by CYC regimen using two-sample independent t-tests or Wilcoxon rank sum tests for continuous variables and Chi-square tests or Fisher's exact tests for categorical variables. A generalized linear model with logit link was fit to evaluate the association of CYC regimen (EL vs. NIH) with renal response. We used a propensity score model and inverse probability weighting to balance clinically important baseline covariates. We adjusted for confounders and precision variables that remained unbalanced in the synthetic dataset.
Results: One hundred and sixty-two patients (97 NIH, 65 EL) were included. Baseline characteristics were similar between groups. EL patients were on average older at the start of current CYC therapy, had longer SLE disease duration and were more likely to have relapsed or refractory LN compared to the NIH group (Table 1). Intravenous (IV) steroid use and mean oral prednisone doses were not significantly different at baseline, 3, 6, and 12 months. The mean eGFR and UPCR improved over the course of study, though the mean UPCR remained >1 (Figure 1). At 12 months, 42% of EL and 53% of NIH treated patients achieved CRR or PRR (p = 0.25), and 19% of EL and 40% of NIH treated patients achieved CRR (p = 0.01). After multivariable adjustment (Table 2), at 12 months there was no significant association between the CYC dosing regimen used and achieving PRR or CRR (OR 0.79, 95% CI 0.34, 1.87) and CRR only (OR 0.80, 95% CI 0.30, 2.11).
Conclusion: Our study found no significant association between CYC regimen (EL vs. NIH) and renal response at 12-months, after multivariable adjustment. However, the study was retrospective and was not powered for non-inferiority. Nevertheless, our data suggest that EL dosing may be a reasonable alternative for treatment of youth with proliferative LN, especially given the potential benefits of lower cumulative CYC exposure. Future prospective studies and longer-term outcome data are needed.
.jpg) Table 1: Baseline Characteristics of the Cohort
Table 1: Baseline Characteristics of the Cohort
.jpg) Table 2. Adjusted Renal Response Outcomes at 12 Months
Table 2. Adjusted Renal Response Outcomes at 12 Months
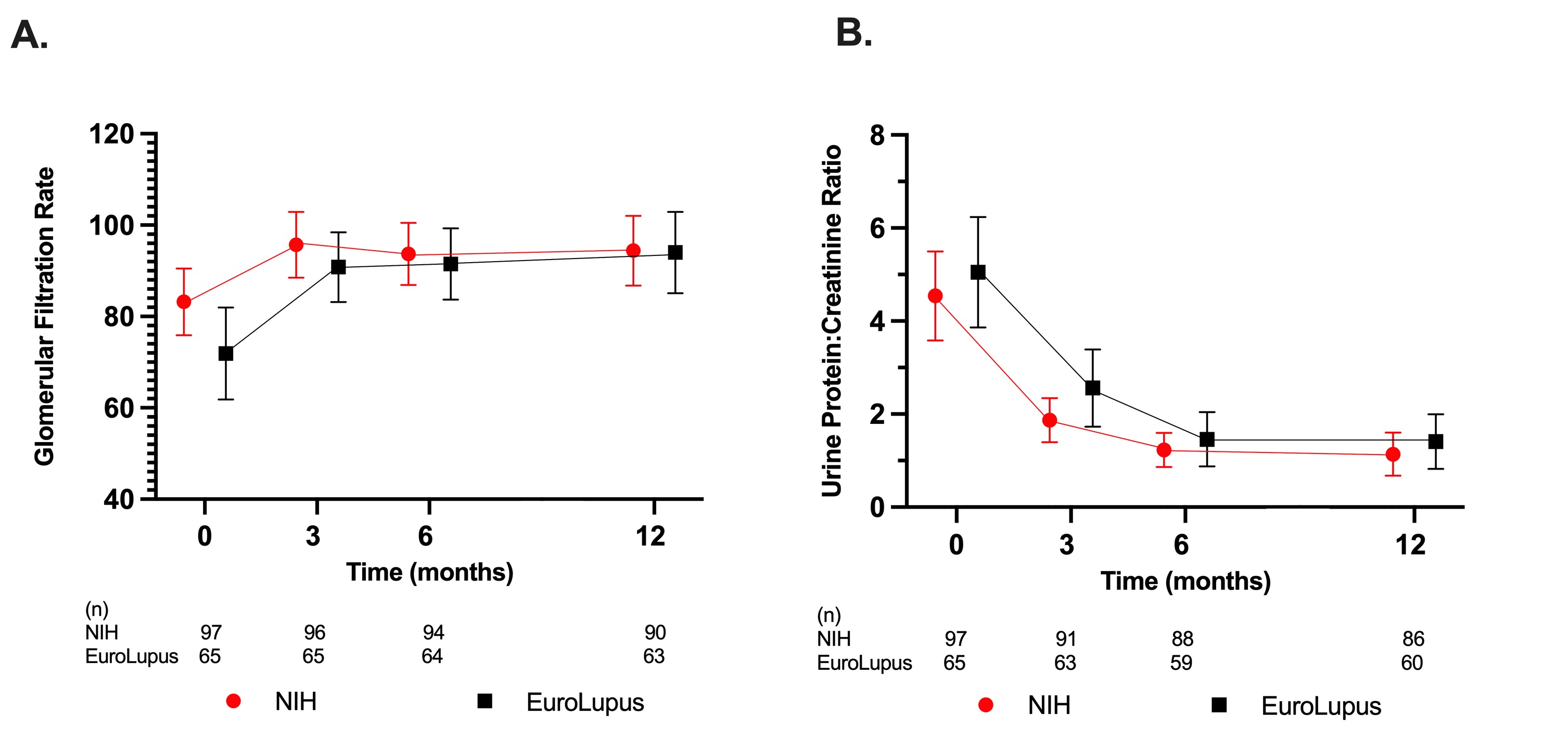 Figure 1. Change in Estimated Glomerular Filtration Rate (eGFR) and Urine Protein-to-Creatinine Ratio (UPCR) Over Time by Dosing Regimen. (A) Mean eGFR (mL/min/per 1.73 m2) over time with 95% confidence interval bands. (B) Mean UPCR (mg protein/mg creatinine) over time, with 95% confidence interval bands.
Figure 1. Change in Estimated Glomerular Filtration Rate (eGFR) and Urine Protein-to-Creatinine Ratio (UPCR) Over Time by Dosing Regimen. (A) Mean eGFR (mL/min/per 1.73 m2) over time with 95% confidence interval bands. (B) Mean UPCR (mg protein/mg creatinine) over time, with 95% confidence interval bands.
Disclosures: C. Wang, None; R. Sadun, None; W. Zhou, None; K. Miller, None; C. Palmer, None; S. Ardoin, None; C. Bacha, None; E. Hause, None; J. Hui-Yuen, None; N. Ling, None; M. Pereira, None; M. Riebschleger, None; K. Rouster-Stevens, Accordant; A. Sarkissian, None; J. Shalen, None; W. Soulsby, None; M. Twilt, None; E. Wu, Pharming Healthcare, Inc, AstraZeneca, Bristol-Myers Squibb(BMS), Janssen; L. Lewandowski, None; S. Wenderfer, Bristol-Myers Squibb(BMS), Alnylam; J. Cooper, None.
Background/Purpose: There have been no published studies comparing renal outcomes of youth with proliferative lupus nephritis (LN) treated with the lower-dose EuroLupus (EL) cyclophosphamide (CYC) regimen vs. the NIH regimen. We aimed to evaluate the association between the CYC dosing regimen and renal response at 12 months.
Methods: A multisite retrospective cohort study was conducted at 11 North American pediatric centers using electronic medical record data. Patients 6 to 22 years of age with active proliferative LN treated with either the EL or NIH regimen from 7/1/2014 to 6/30/2021 were included. Data were obtained through 12 months after baseline (defined as start of most recent CYC course). The primary outcome was the proportion of patients with complete (CRR) or partial renal response (PRR) at 12 months. The secondary outcome was the proportion of patients with CRR at 12 months. CRR was defined as normal estimated glomerular filtration rate (eGFR > 90 mL/min/1.72 m2) and urine protein-to-creatinine ratio (UPCR) < 0.2. PRR was defined as ≥ 50% improvement in eGFR and UPCR if abnormal at baseline and UPCR ≤ 1. Urine sediment was excluded from response definitions due to inability to exclude concurrent menses (hematuria), infection (pyuria) and missing values (casts). Baseline characteristics were compared by CYC regimen using two-sample independent t-tests or Wilcoxon rank sum tests for continuous variables and Chi-square tests or Fisher's exact tests for categorical variables. A generalized linear model with logit link was fit to evaluate the association of CYC regimen (EL vs. NIH) with renal response. We used a propensity score model and inverse probability weighting to balance clinically important baseline covariates. We adjusted for confounders and precision variables that remained unbalanced in the synthetic dataset.
Results: One hundred and sixty-two patients (97 NIH, 65 EL) were included. Baseline characteristics were similar between groups. EL patients were on average older at the start of current CYC therapy, had longer SLE disease duration and were more likely to have relapsed or refractory LN compared to the NIH group (Table 1). Intravenous (IV) steroid use and mean oral prednisone doses were not significantly different at baseline, 3, 6, and 12 months. The mean eGFR and UPCR improved over the course of study, though the mean UPCR remained >1 (Figure 1). At 12 months, 42% of EL and 53% of NIH treated patients achieved CRR or PRR (p = 0.25), and 19% of EL and 40% of NIH treated patients achieved CRR (p = 0.01). After multivariable adjustment (Table 2), at 12 months there was no significant association between the CYC dosing regimen used and achieving PRR or CRR (OR 0.79, 95% CI 0.34, 1.87) and CRR only (OR 0.80, 95% CI 0.30, 2.11).
Conclusion: Our study found no significant association between CYC regimen (EL vs. NIH) and renal response at 12-months, after multivariable adjustment. However, the study was retrospective and was not powered for non-inferiority. Nevertheless, our data suggest that EL dosing may be a reasonable alternative for treatment of youth with proliferative LN, especially given the potential benefits of lower cumulative CYC exposure. Future prospective studies and longer-term outcome data are needed.
.jpg) Table 1: Baseline Characteristics of the Cohort
Table 1: Baseline Characteristics of the Cohort.jpg) Table 2. Adjusted Renal Response Outcomes at 12 Months
Table 2. Adjusted Renal Response Outcomes at 12 Months Figure 1. Change in Estimated Glomerular Filtration Rate (eGFR) and Urine Protein-to-Creatinine Ratio (UPCR) Over Time by Dosing Regimen. (A) Mean eGFR (mL/min/per 1.73 m2) over time with 95% confidence interval bands. (B) Mean UPCR (mg protein/mg creatinine) over time, with 95% confidence interval bands.
Figure 1. Change in Estimated Glomerular Filtration Rate (eGFR) and Urine Protein-to-Creatinine Ratio (UPCR) Over Time by Dosing Regimen. (A) Mean eGFR (mL/min/per 1.73 m2) over time with 95% confidence interval bands. (B) Mean UPCR (mg protein/mg creatinine) over time, with 95% confidence interval bands. Disclosures: C. Wang, None; R. Sadun, None; W. Zhou, None; K. Miller, None; C. Palmer, None; S. Ardoin, None; C. Bacha, None; E. Hause, None; J. Hui-Yuen, None; N. Ling, None; M. Pereira, None; M. Riebschleger, None; K. Rouster-Stevens, Accordant; A. Sarkissian, None; J. Shalen, None; W. Soulsby, None; M. Twilt, None; E. Wu, Pharming Healthcare, Inc, AstraZeneca, Bristol-Myers Squibb(BMS), Janssen; L. Lewandowski, None; S. Wenderfer, Bristol-Myers Squibb(BMS), Alnylam; J. Cooper, None.

