Back
Abstract Session
Session: Abstracts: Miscellaneous Rheumatic and Inflammatory Diseases I (0506–0511)
0509: Guselkumab Provides Sustained Improvements in Work Productivity and Daily Activity in Patients with Active Psoriatic Arthritis Through 2 Years of DISCOVER-2
Saturday, November 12, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 119

Jeffrey Curtis, MD, MS, MPH
University of Alabama at Birmingham
Hoover, AL, United States
Presenting Author(s)
Jeffrey Curtis1, Iain B McInnes2, Proton Rahman3, Dafna Gladman4, Feifei Yang5, Steve Peterson5, Alexa Kollmeier6, Natalie Shiff7, Chenglong Han8, May Shawi9, William Tillett10 and Philip J Mease11, 1Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, Birmingham, AL, 2Institute of Infection, Immunity and Inflammation, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, Scotland, United Kingdom, 3Memorial University, St. John's, NL, Canada, 4Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 5Department of Immunology, Janssen Global Services, LLC, Horsham, PA, 6Janssen-Cilag, Research & Development, LLC, San Diego, CA, 7Janssen Scientific Affairs, LLC; Community Health and Epidemiology, University of Saskatchewan, Philadelphia, PA, 8Immunology, Janssen Research & Development, LLC, Spring House, PA, 9Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, Horsham, PA, 10Royal National Hospital for Rheumatic Diseases, Bath, United Kingdom, 11Swedish Medical Center/Providence St. Joseph Health, Seattle, WA
Background/Purpose: Psoriatic arthritis (PsA) impacts patients' work productivity (WP) and daily activity.1 DISCOVER-2 (D2), a phase 3 trial of the selective interleukin-23 p19-subunit inhibitor guselkumab (GUS) in biologic-naïve patients with PsA,2 demonstrated significant improvements in patient-reported WP and daily activity following 1 year of GUS treatment.3 This study assessed WP and daily activity impairment in D2 patients through 2 years, estimated indirect savings associated with GUS treatment, and assessed changes in employment status.
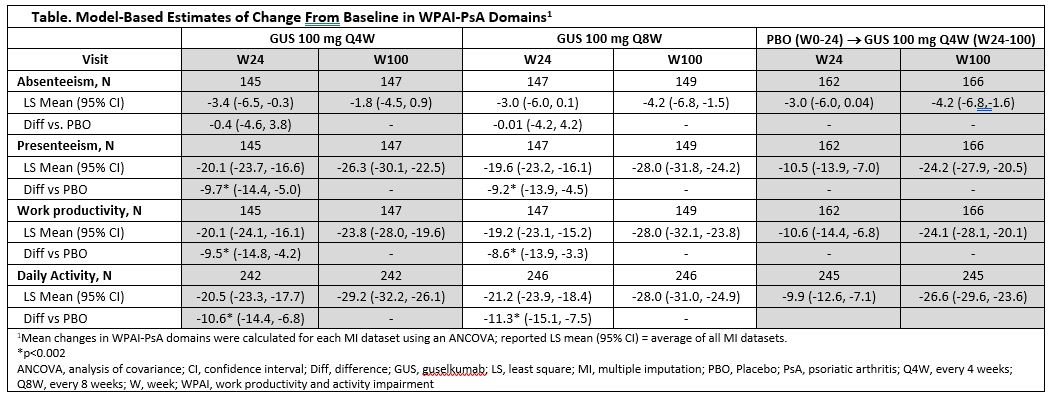
Methods: Patients with active PsA received GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at Week (W) 0, W4, then Q8W; or placebo (PBO). At W24, PBO patients crossed over to GUS 100 mg Q4W. Work productivity and activity impairment (WPAI)-PsA assesses PsA-related work time missed (absenteeism), impairment while working (presenteeism), and impaired overall WP (absenteeism + presenteeism) for patients employed at baseline (EBL) and daily activity for all patients, including those unemployed at baseline (UBL) during the previous week. Mean changes in WPAI-PsA domains were calculated for each multiple imputation (MI) dataset using an analysis of covariance (ANCOVA); the reported least square (LS) mean is the average of all MI datasets. Significance was defined as p< 0.05. Among patients EBL, potential indirect savings from improved overall WP were estimated using 2020 European Union mean yearly wage estimate (all occupations) combined with LS mean change from BL in WPAI-PsA overall work impairment.4 A shift analysis evaluated proportions of patients employed vs unemployed by treatment group using observed data over time.
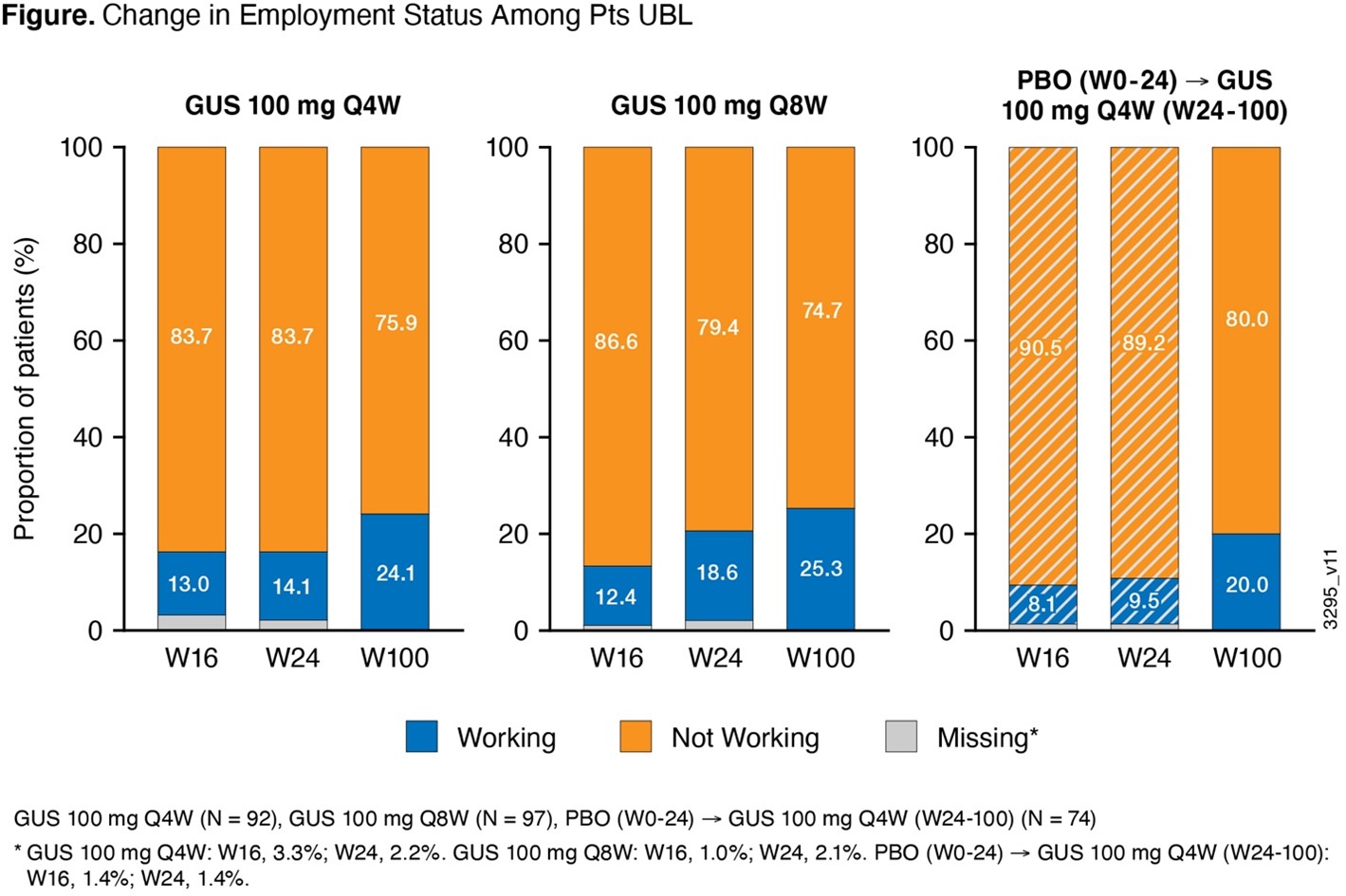
Results: Patients EBL comprised 64% of the analysis cohort. Significant improvements in WP in patients EBL and in daily activity among all patients were observed with GUS Q4W/Q8W vs PBO at W24;3 mean improvements in WP and daily activity increased with continued GUS through 2 years (Table). Potential annual indirect savings from improved overall WP in patients EBL were €10,826 GUS Q4W, €12,712 GUS Q8W, and €10,948 PBO® GUS Q4W at 2 years. Shift analysis showed relatively stable employment in patients EBL with GUS up to 2 years ( >83% continued to work). Among patients UBL (36% of cohort), the proportion of patients employed increased by >20% through 2 years of GUS (Figure).
Conclusion: In GUS-treated bio-naïve PsA patients, robust improvements in WP and daily activity seen at W24 were maintained and increased through 2 years of GUS. Long-term improvements in WP achieved may result in substantial indirect cost savings for GUS-treated patients. Rates of employment remained stable in patients employed and increased in those unemployed at BL.
References:
1. Tillett W et al. Rheumatol (Oxford). 2012;51:275–83.
2. Mease PJ et al. Lancet. 2020;395:1126–36.
3. Curtis JR et al. EULAR, June 2–5, 2021. POS1026.
4. OECD (2020). Average wages (indicator). https://data.oecd.org/earnwage/average-wages.htm
 GUS, guselkumab; PBO, placebo; pts, patients; Q4W, every 4 weeks; Q8W, every 8 weeks; UBL, unemployed at baseline; W, week
GUS, guselkumab; PBO, placebo; pts, patients; Q4W, every 4 weeks; Q8W, every 8 weeks; UBL, unemployed at baseline; W, week
Disclosures: J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; I. McInnes, Bristol-Myers Squibb (BMS), Janssen, Novartis, UCB, Pfizer, AbbVie, Celgene, AstraZeneca, Boehringer Ingelheim, EveloBio, LEO, Lilly; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; F. Yang, Janssen Global Services, LLC; S. Peterson, Johnson & Johnson; A. Kollmeier, Janssen Research & Development, LLC, Johnson & Johnson; N. Shiff, AbbVie/Abbott, Gilead, Johnson & Johnson, Janssen Scientific Affairs, LLC; C. Han, Janssen Research and Development, LLC, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.
Background/Purpose: Psoriatic arthritis (PsA) impacts patients' work productivity (WP) and daily activity.1 DISCOVER-2 (D2), a phase 3 trial of the selective interleukin-23 p19-subunit inhibitor guselkumab (GUS) in biologic-naïve patients with PsA,2 demonstrated significant improvements in patient-reported WP and daily activity following 1 year of GUS treatment.3 This study assessed WP and daily activity impairment in D2 patients through 2 years, estimated indirect savings associated with GUS treatment, and assessed changes in employment status.
Methods: Patients with active PsA received GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at Week (W) 0, W4, then Q8W; or placebo (PBO). At W24, PBO patients crossed over to GUS 100 mg Q4W. Work productivity and activity impairment (WPAI)-PsA assesses PsA-related work time missed (absenteeism), impairment while working (presenteeism), and impaired overall WP (absenteeism + presenteeism) for patients employed at baseline (EBL) and daily activity for all patients, including those unemployed at baseline (UBL) during the previous week. Mean changes in WPAI-PsA domains were calculated for each multiple imputation (MI) dataset using an analysis of covariance (ANCOVA); the reported least square (LS) mean is the average of all MI datasets. Significance was defined as p< 0.05. Among patients EBL, potential indirect savings from improved overall WP were estimated using 2020 European Union mean yearly wage estimate (all occupations) combined with LS mean change from BL in WPAI-PsA overall work impairment.4 A shift analysis evaluated proportions of patients employed vs unemployed by treatment group using observed data over time.
Results: Patients EBL comprised 64% of the analysis cohort. Significant improvements in WP in patients EBL and in daily activity among all patients were observed with GUS Q4W/Q8W vs PBO at W24;3 mean improvements in WP and daily activity increased with continued GUS through 2 years (Table). Potential annual indirect savings from improved overall WP in patients EBL were €10,826 GUS Q4W, €12,712 GUS Q8W, and €10,948 PBO® GUS Q4W at 2 years. Shift analysis showed relatively stable employment in patients EBL with GUS up to 2 years ( >83% continued to work). Among patients UBL (36% of cohort), the proportion of patients employed increased by >20% through 2 years of GUS (Figure).
Conclusion: In GUS-treated bio-naïve PsA patients, robust improvements in WP and daily activity seen at W24 were maintained and increased through 2 years of GUS. Long-term improvements in WP achieved may result in substantial indirect cost savings for GUS-treated patients. Rates of employment remained stable in patients employed and increased in those unemployed at BL.
References:
1. Tillett W et al. Rheumatol (Oxford). 2012;51:275–83.
2. Mease PJ et al. Lancet. 2020;395:1126–36.
3. Curtis JR et al. EULAR, June 2–5, 2021. POS1026.
4. OECD (2020). Average wages (indicator). https://data.oecd.org/earnwage/average-wages.htm

 GUS, guselkumab; PBO, placebo; pts, patients; Q4W, every 4 weeks; Q8W, every 8 weeks; UBL, unemployed at baseline; W, week
GUS, guselkumab; PBO, placebo; pts, patients; Q4W, every 4 weeks; Q8W, every 8 weeks; UBL, unemployed at baseline; W, weekDisclosures: J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; I. McInnes, Bristol-Myers Squibb (BMS), Janssen, Novartis, UCB, Pfizer, AbbVie, Celgene, AstraZeneca, Boehringer Ingelheim, EveloBio, LEO, Lilly; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; F. Yang, Janssen Global Services, LLC; S. Peterson, Johnson & Johnson; A. Kollmeier, Janssen Research & Development, LLC, Johnson & Johnson; N. Shiff, AbbVie/Abbott, Gilead, Johnson & Johnson, Janssen Scientific Affairs, LLC; C. Han, Janssen Research and Development, LLC, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.

