Back
Poster Session D
Immunobiology
Session: (1681–1706) Innate Immunity Poster: Basic and Translational Science
1701: High TNFAIP3-Transfected Cell Activity: An In Vitro Model for Phagocyte Study of Monogenic Behçet’s Disease Pathophysiology
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- PP
Patricia Pontes Aires, MD
Universidade Federal de Sao Paulo
São Paulo, S�o Paulo, Brazil
Abstract Poster Presenter(s)
Patricia Pontes Aires1, André Cunha1, DANIELA GERENT PETRY PIOTTO1, Maria Teresa Terreri2 and Sandro Perazzio1, 1Universidade Federal de São Paulo, São Paulo, Brazil, 2Universidad Federal São Paulo, São Paulo, Brazil
Background/Purpose: A20 haploinsufficiency (A20HA), an important monogenic etiology of Behçet's disease (BD), is caused by loss-of-function mutations in TNFAIP3. Although monocytes play an important role on BD pathophysiology, the effect of TNFAIP3 mutations in these cells needs further elucidation. Our group previously described high soluble CD40 ligand (sCD40L) serum levels, which induced phagocyte hyperactivity. This study aimed to assess different phagocyte function of THP-1 (monocytic) cells transfected with the pathogenic TNFAIP3 variant L227X.
Methods: THP-1 or HL-60 (neutrophilic) cells were transfected with a GFP-tagged plasmid containing either TNFAIP3 or mutated TNFAIP3-L227X gene. Flow cytometry analysis before and after stimulus with sCD40L, monosodium urate crystals (MSU) or phorbol-miristate-acetate (PMA): 1) phagocytic activity was assessed using fluorescent zymosan particles; 2) CellRox Deep Red assessed reactive oxygen species (ROS) production; 3) Ki-67 expression quantified cell proliferation.
Results: THP-1-TNFAIP3-L227X [mean of fluorescence intensity (MFI) 94748±28804] showed higher phagocytic activity than THP-1 (34039.33±4945; p< 0.001) and THP-1-TNFAIP3 (18476±379; p< 0.001) at baseline and after sCD40L stimulus (respectively, 76265±15858 vs 35835±1133 vs 25379±3472; p< 0.05; Figure 1). Similarly, HL-60-TNFAIP3-L227X cells (17480±2306) showed higher phagocytic activity than HL-60 (5862±410; p< 0.01) at baseline and after MSU stimulus (16869±3496 vs 5482±324; p< 0.01). Conversely, percentage of phagocytosing THP-1-TNFAIP3-L227X cells (13.3%±2.6%) was lower than THP-1-TNFAIP3 (23.7±2.5%, p< 0.01); on the other hand, there was no statistical difference in the percentage of HL-60-TNFAIP3-L227X phagocytosing cells, compared to HL-60-TNFAIP3. Constitutional ROS production by THP-1-TNFAIP3 cells (MFI=291919±27871) was higher than THP-1 (98226±7285; p< 0.001) and maintained after PMA (561333±84760 vs 399628±69821; p< 0.001; Figure 2). ROS production by THP-1-TNFAIP3 was also higher than THP-1-TNFAIP3-L227X (230711+7895, p< 0.001). Despite THP-1 typical arrest in G2 phase, PMA-induced Ki-67 expression of THP-1-TNFAIP3-L227X was significantly increased (19184±8934) compared with THP-1 (2434±353) and THP-1-TNFAIP3 (4463±357; p< 0.01; Figure 3).
Conclusion: THP-1-TNFAIP3-L227X presented higher phagocytic activity, a pattern also observed by neutrophilic cells (HL-60-TNFAIP3-L227X), suggesting a specific mutation-induced phenotype. Paradoxical higher MFI of THP-1-TNFAIP3-L227X with lower percentage cell number could be explained by phagocytosis of multiple zymosan particles by a single cell. Decreased THP-1-TNFAIP3-L227X oxidative burst suggests a state of cell exhaustion. Higher PMA-induced THP-1TNFAIP3-L227X proliferation could eventually be explored by functional studies monogenic BD patients. THP-1-TNFAIP3 proliferation rates were low and unchanged upon stimulation, reinforcing the role of A20 as an NF-κB pathway inhibitor.
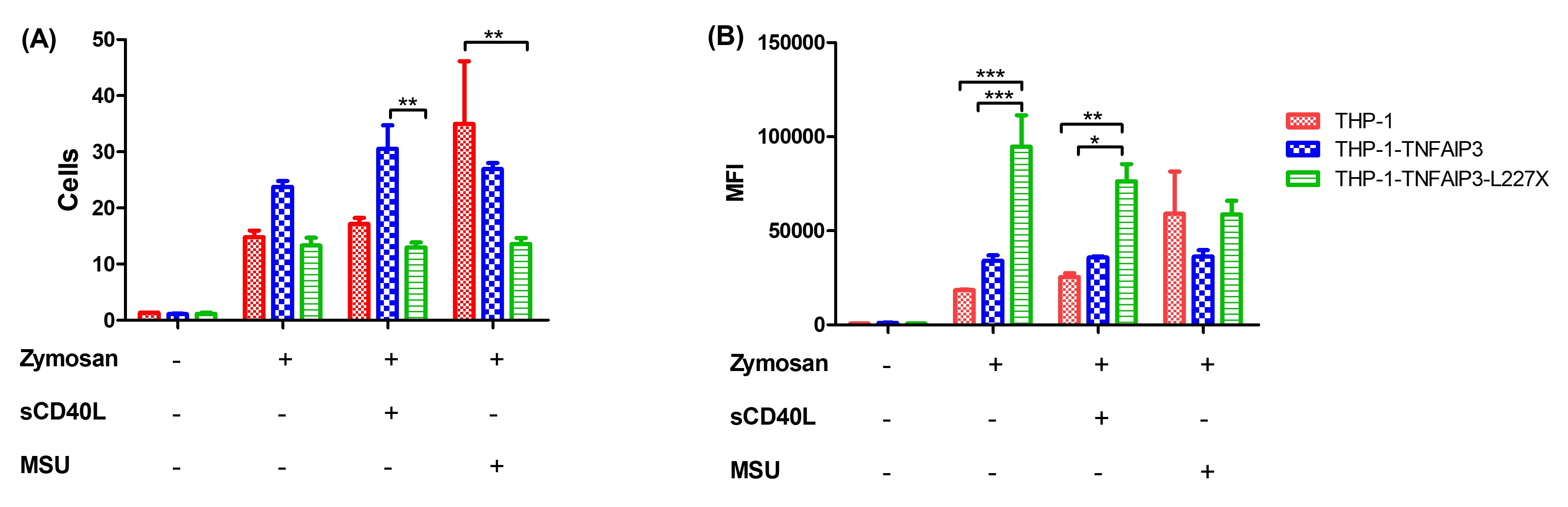 Figure 1: phagocytosis of Alexa-Fluor 594-tagged zymosan particles by THP-1 cells. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results indicate higher phagocytic rates for THP-1-TNFAIP3-L227X at baseline and upon stimulation with sCD40L * p < 0.05; ** p < 0.01; *** p < 0.001
Figure 1: phagocytosis of Alexa-Fluor 594-tagged zymosan particles by THP-1 cells. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results indicate higher phagocytic rates for THP-1-TNFAIP3-L227X at baseline and upon stimulation with sCD40L * p < 0.05; ** p < 0.01; *** p < 0.001
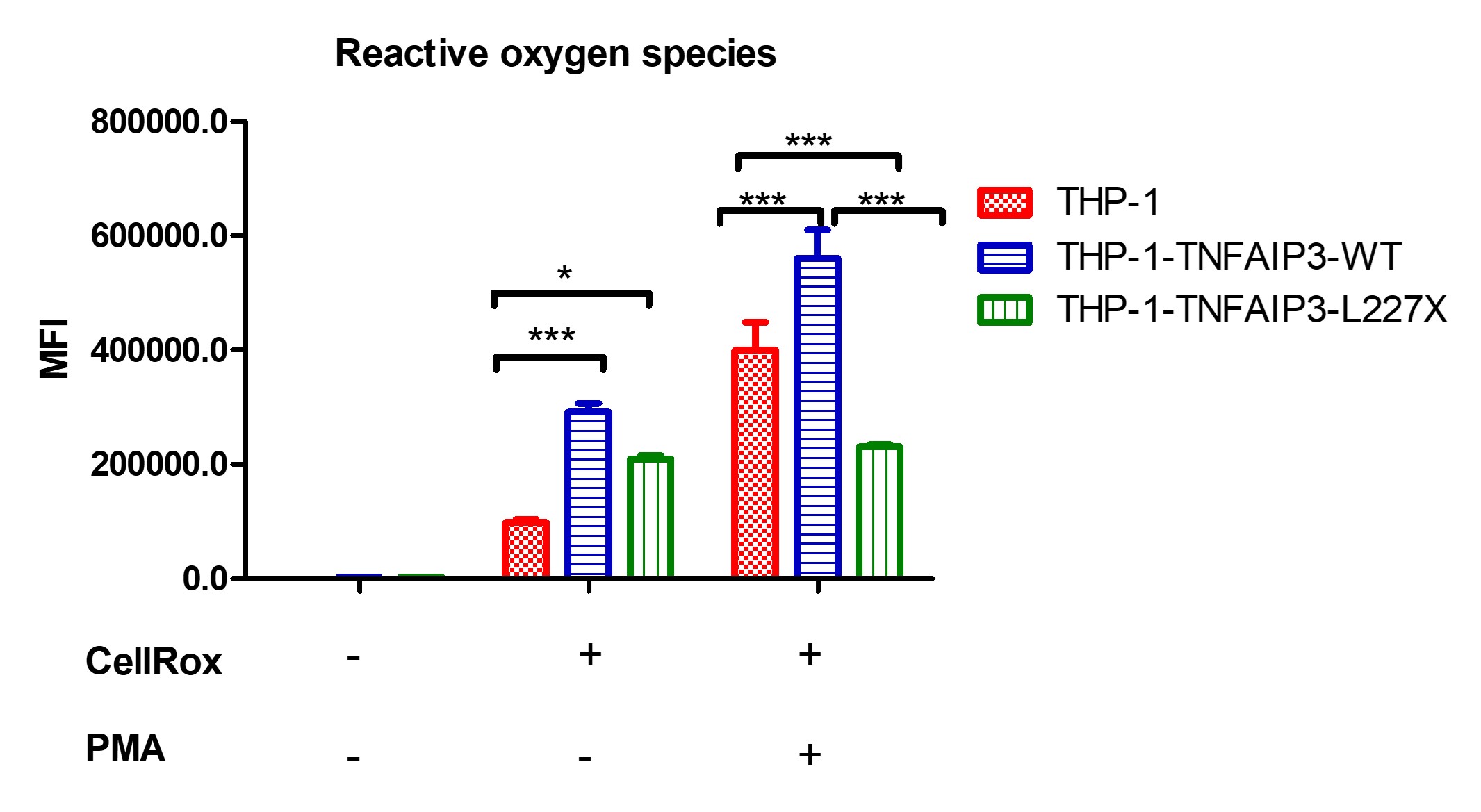 Figure 2: production of ROS by THP-1 cells, before and after PMA stimulus. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results indicate higher oxidative burst in THP-1-TNFAIP3-WT cells and unresponsiveness of THP-1-TNFAIP3-L227X, which could indicate a state of cell exhaustion. * p < 0.05; *** p < 0.001
Figure 2: production of ROS by THP-1 cells, before and after PMA stimulus. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results indicate higher oxidative burst in THP-1-TNFAIP3-WT cells and unresponsiveness of THP-1-TNFAIP3-L227X, which could indicate a state of cell exhaustion. * p < 0.05; *** p < 0.001
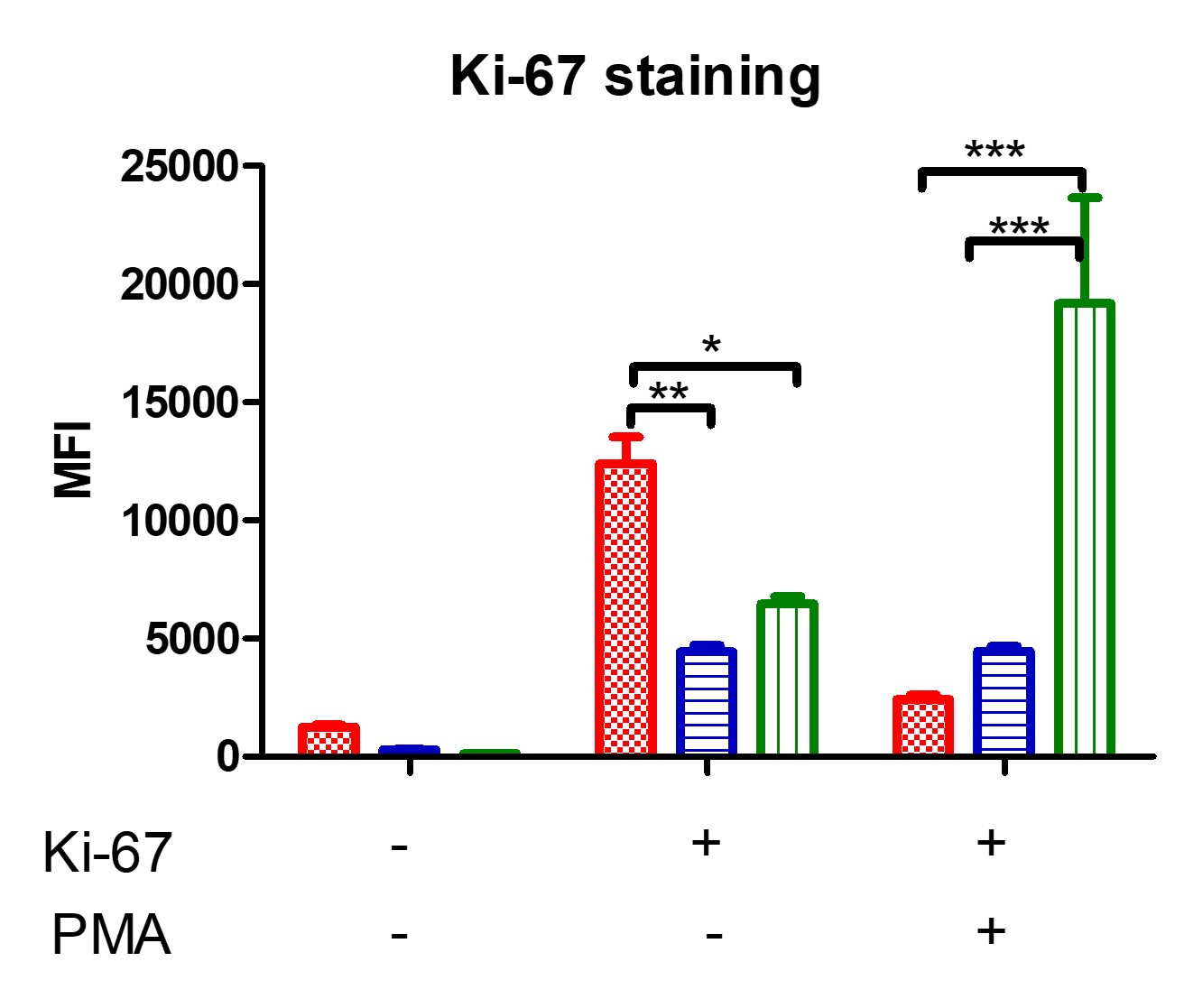 Figure 3: analysis of THP-1 cell proliferation using flow cytometry, through Ki-67 staining, before and after an 18-hour incubation with PMA. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results show higher proliferation rates for THP-1-TNFAIP3-L227X and low proliferation rates for THP-1-TNFAIP3-WT. * p < 0.05; ** p < 0.01; *** p < 0.001
Figure 3: analysis of THP-1 cell proliferation using flow cytometry, through Ki-67 staining, before and after an 18-hour incubation with PMA. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results show higher proliferation rates for THP-1-TNFAIP3-L227X and low proliferation rates for THP-1-TNFAIP3-WT. * p < 0.05; ** p < 0.01; *** p < 0.001
Disclosures: P. Pontes Aires, None; A. Cunha, None; D. PIOTTO, None; M. Terreri, Roche, Pfizer, UCB, Janssen, Bristol-Myers Squibb(BMS), Eli Lilly, AbbVie/Abbott; S. Perazzio, None.
Background/Purpose: A20 haploinsufficiency (A20HA), an important monogenic etiology of Behçet's disease (BD), is caused by loss-of-function mutations in TNFAIP3. Although monocytes play an important role on BD pathophysiology, the effect of TNFAIP3 mutations in these cells needs further elucidation. Our group previously described high soluble CD40 ligand (sCD40L) serum levels, which induced phagocyte hyperactivity. This study aimed to assess different phagocyte function of THP-1 (monocytic) cells transfected with the pathogenic TNFAIP3 variant L227X.
Methods: THP-1 or HL-60 (neutrophilic) cells were transfected with a GFP-tagged plasmid containing either TNFAIP3 or mutated TNFAIP3-L227X gene. Flow cytometry analysis before and after stimulus with sCD40L, monosodium urate crystals (MSU) or phorbol-miristate-acetate (PMA): 1) phagocytic activity was assessed using fluorescent zymosan particles; 2) CellRox Deep Red assessed reactive oxygen species (ROS) production; 3) Ki-67 expression quantified cell proliferation.
Results: THP-1-TNFAIP3-L227X [mean of fluorescence intensity (MFI) 94748±28804] showed higher phagocytic activity than THP-1 (34039.33±4945; p< 0.001) and THP-1-TNFAIP3 (18476±379; p< 0.001) at baseline and after sCD40L stimulus (respectively, 76265±15858 vs 35835±1133 vs 25379±3472; p< 0.05; Figure 1). Similarly, HL-60-TNFAIP3-L227X cells (17480±2306) showed higher phagocytic activity than HL-60 (5862±410; p< 0.01) at baseline and after MSU stimulus (16869±3496 vs 5482±324; p< 0.01). Conversely, percentage of phagocytosing THP-1-TNFAIP3-L227X cells (13.3%±2.6%) was lower than THP-1-TNFAIP3 (23.7±2.5%, p< 0.01); on the other hand, there was no statistical difference in the percentage of HL-60-TNFAIP3-L227X phagocytosing cells, compared to HL-60-TNFAIP3. Constitutional ROS production by THP-1-TNFAIP3 cells (MFI=291919±27871) was higher than THP-1 (98226±7285; p< 0.001) and maintained after PMA (561333±84760 vs 399628±69821; p< 0.001; Figure 2). ROS production by THP-1-TNFAIP3 was also higher than THP-1-TNFAIP3-L227X (230711+7895, p< 0.001). Despite THP-1 typical arrest in G2 phase, PMA-induced Ki-67 expression of THP-1-TNFAIP3-L227X was significantly increased (19184±8934) compared with THP-1 (2434±353) and THP-1-TNFAIP3 (4463±357; p< 0.01; Figure 3).
Conclusion: THP-1-TNFAIP3-L227X presented higher phagocytic activity, a pattern also observed by neutrophilic cells (HL-60-TNFAIP3-L227X), suggesting a specific mutation-induced phenotype. Paradoxical higher MFI of THP-1-TNFAIP3-L227X with lower percentage cell number could be explained by phagocytosis of multiple zymosan particles by a single cell. Decreased THP-1-TNFAIP3-L227X oxidative burst suggests a state of cell exhaustion. Higher PMA-induced THP-1TNFAIP3-L227X proliferation could eventually be explored by functional studies monogenic BD patients. THP-1-TNFAIP3 proliferation rates were low and unchanged upon stimulation, reinforcing the role of A20 as an NF-κB pathway inhibitor.
 Figure 1: phagocytosis of Alexa-Fluor 594-tagged zymosan particles by THP-1 cells. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results indicate higher phagocytic rates for THP-1-TNFAIP3-L227X at baseline and upon stimulation with sCD40L * p < 0.05; ** p < 0.01; *** p < 0.001
Figure 1: phagocytosis of Alexa-Fluor 594-tagged zymosan particles by THP-1 cells. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results indicate higher phagocytic rates for THP-1-TNFAIP3-L227X at baseline and upon stimulation with sCD40L * p < 0.05; ** p < 0.01; *** p < 0.001 Figure 2: production of ROS by THP-1 cells, before and after PMA stimulus. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results indicate higher oxidative burst in THP-1-TNFAIP3-WT cells and unresponsiveness of THP-1-TNFAIP3-L227X, which could indicate a state of cell exhaustion. * p < 0.05; *** p < 0.001
Figure 2: production of ROS by THP-1 cells, before and after PMA stimulus. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results indicate higher oxidative burst in THP-1-TNFAIP3-WT cells and unresponsiveness of THP-1-TNFAIP3-L227X, which could indicate a state of cell exhaustion. * p < 0.05; *** p < 0.001 Figure 3: analysis of THP-1 cell proliferation using flow cytometry, through Ki-67 staining, before and after an 18-hour incubation with PMA. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results show higher proliferation rates for THP-1-TNFAIP3-L227X and low proliferation rates for THP-1-TNFAIP3-WT. * p < 0.05; ** p < 0.01; *** p < 0.001
Figure 3: analysis of THP-1 cell proliferation using flow cytometry, through Ki-67 staining, before and after an 18-hour incubation with PMA. Comparison of THP-1, THP-1-TNFAIP3-WT and THP-1-TNFAIP3-L227X cells, in triplicate, using two-way ANOVA. Results show higher proliferation rates for THP-1-TNFAIP3-L227X and low proliferation rates for THP-1-TNFAIP3-WT. * p < 0.05; ** p < 0.01; *** p < 0.001Disclosures: P. Pontes Aires, None; A. Cunha, None; D. PIOTTO, None; M. Terreri, Roche, Pfizer, UCB, Janssen, Bristol-Myers Squibb(BMS), Eli Lilly, AbbVie/Abbott; S. Perazzio, None.

