Back
Poster Session D
Session: (2108–2153) Spondyloarthritis Including PsA – Treatment Poster III: PsA
2113: Improvement in Key PsA Core Domains with Guselkumab Treatment in an Enriched Population of ACR20 Non-Responders at Week 24: Post Hoc Analysis of Two Phase 3 Studies
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
- DM
Dennis McGonagle, MD, PhD
Uni of Leeds
Leeds, United Kingdom
Abstract Poster Presenter(s)
Dennis McGonagle1, Derek Haaland2, Philip Helliwell3, A. Marilise Marrache4, May Shawi5, Emmanouil Rampakakis6, Peter Nash7, Fedra Irazoque Palazuelos8 and Arthur Kavanaugh9, 1Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds; National Institute for Health Research, Leeds Biomedical Research Centre, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 2The Waterside Clinic, Oro Medonte, ON, Canada, 3Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom, 4Janssen Inc., Dollard-des-Ormeaux, QC, Canada, 5Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, Horsham, PA, 6McGill University, Department of Pediatrics and JSS Medical Research, Montréal, QC, Canada, 7School of Medicine, Griffith University, Sunshine Coast, Australia, 8Angeles Mocel Hospital; Universidad Autónoma de México, Ciudad de México, Mexico, 9University of California San Diego, La Jolla, CA
Background/Purpose: Despite available PsA treatments, a portion of PsA patients (pts) does not achieve improvements in PsA signs and symptoms according to ACR response criteria. Using data from the phase 3 DISCOVER-1 and DISCOVER-2 (D1/D2) studies in pts with active PsA, the purpose of this analysis was to describe the benefit of the IL-23p19 inhibitor, guselkumab (GUS), vs placebo (PBO) across key PsA domains, including those not comprising the core ACR measures, in pts not meeting ACR response criteria.
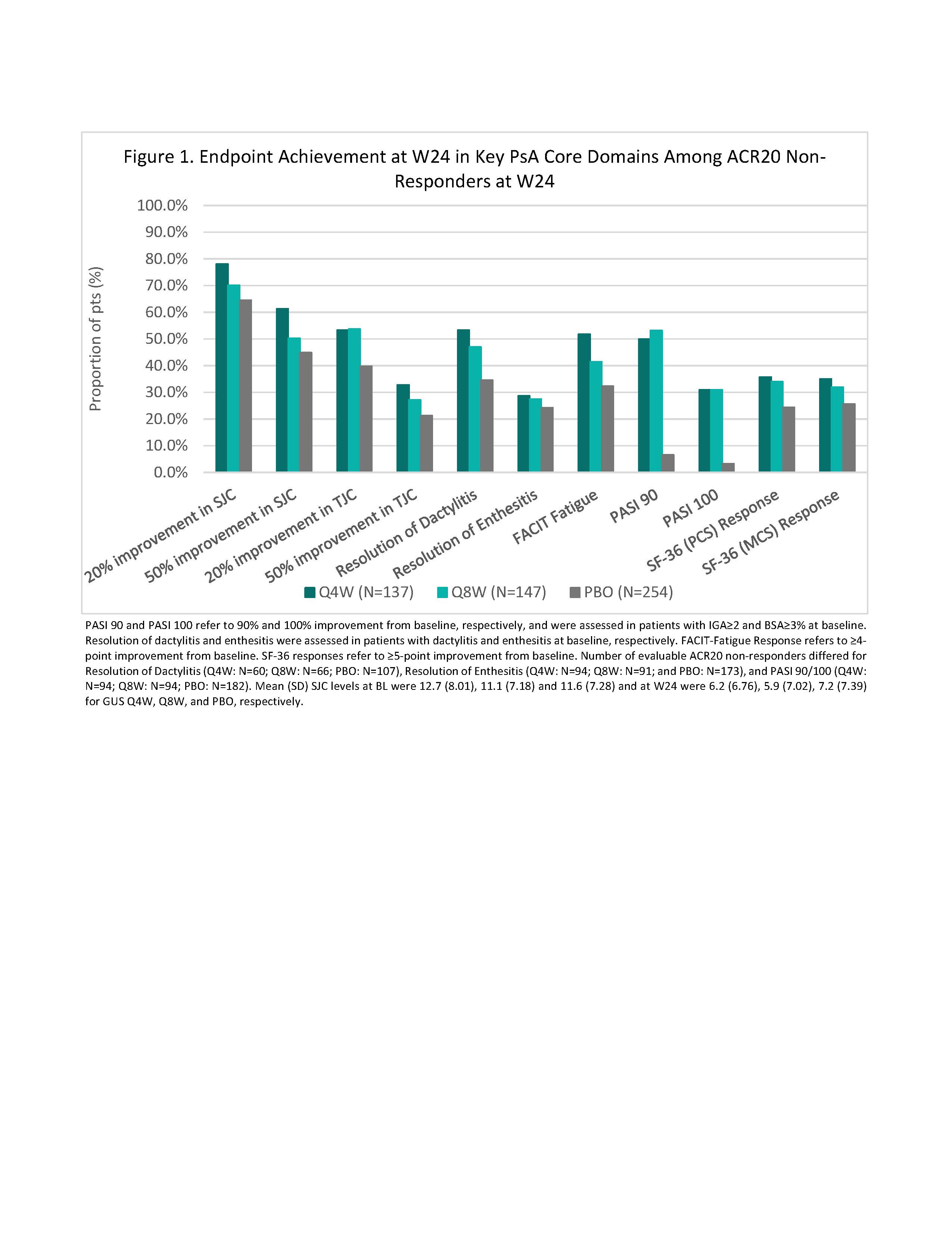
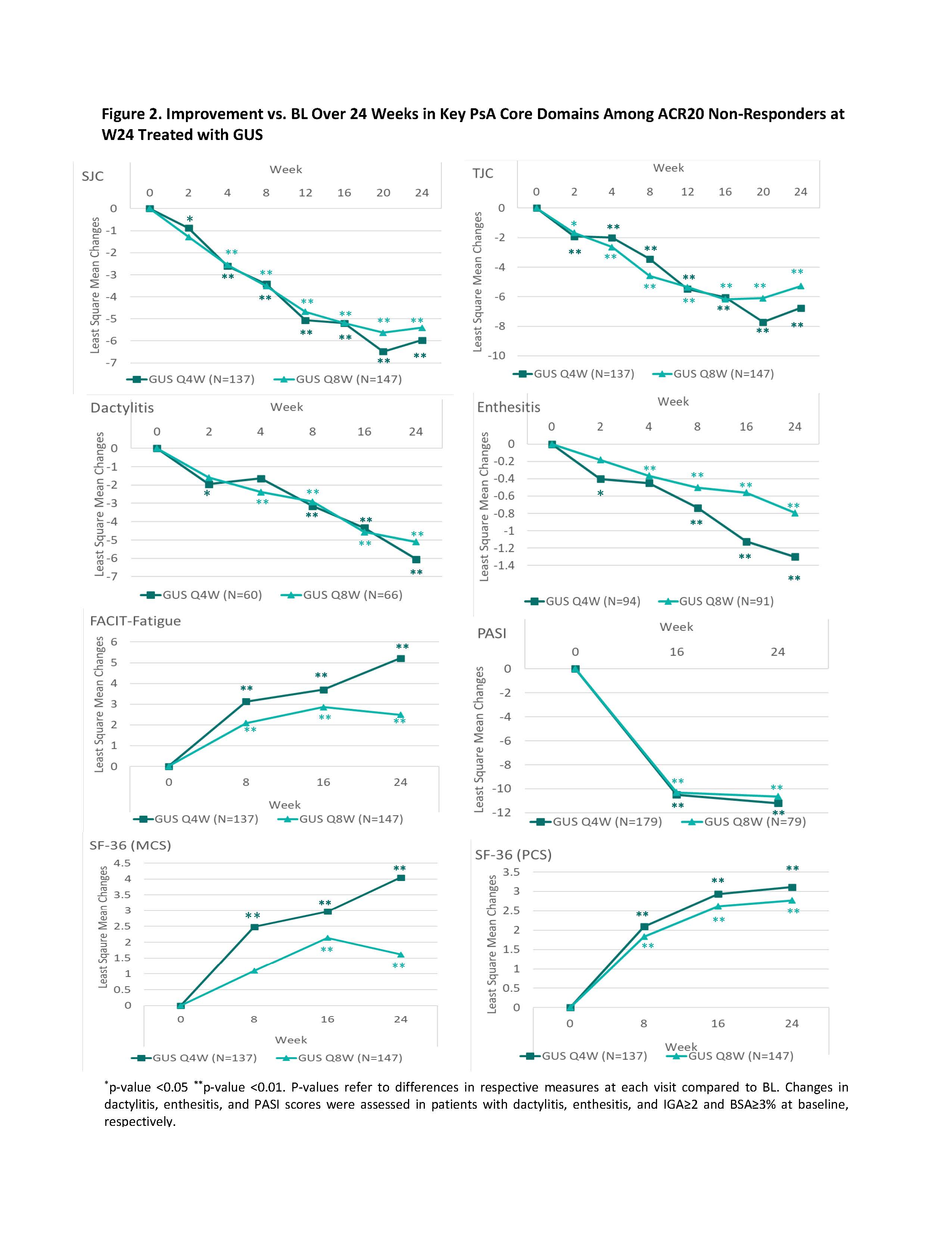
Methods: Pts enrolled in D1 and D2 were adults with active PsA despite standard therapies. D1 pts had ≥3 swollen and ≥3 tender joints (SJC; TJC) and CRP ≥0.3 mg/dL; D2 pts had SJC ≥5, TJC ≥5 TJC, and CRP ≥0.6 mg/dL. 31% of D1 pts received 1-2 prior tumor necrosis factor inhibitors (TNFi); D2 pts were biologic-naïve. Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or PBO; PBO pts crossed over to GUS 100 mg Q4W at W24. In this post hoc analysis, patients not meeting ≥20% improvement in ACR response criteria (ACR20) at W24 were included. Changes from baseline (BL) over 24 W in continuous outcomes of interest (SJC; TJC; enthesitis/dactylitis scores; psoriasis area and severity index [PASI] score; Functional-Assessment of Chronic Illness Therapy [FACIT]-fatigue score; and SF-36 physical [PCS] & mental [MCS] component summary scores) were assessed with repeated measures mixed models adjusting for treatment group, baseline non-biologic DMARD use, and prior TNFi use. Descriptive statistics were produced for categorical outcomes at W24 (Figure 1) using non-responder imputation for missing data.
Results: Of the 1120 pooled D1/D2 pts, 538 did into achieve an ACR20 response at W24, including 137 (36.7%) GUS Q4W, 147 (39.2%) GUS Q8W, and 254 (68.3%) PBO pts, and were included in this analysis. A consistently greater proportion of GUS- than PBO-treated pts achieved W24 categorical outcomes, including those relating to skin, tender joints, and dactylitis, with similar findings for GUS Q4W and Q8W (Figure 1). For continuous endpoints, the benefit of GUS was seen as early as W2 for SJC (W4 for Q8W), TJC, dactylitis (W4 for Q8W), and enthesitis (W4 for Q8W); W8 for FACIT-fatigue, SF-36 PCS, and SF-36 MCS (W16 for Q8W); and W16 for PASI; most endpoints continued to improve through W24 (Figure 2). Across both GUS treatment groups, 43.5%-48.9% of W24 ACR20 non-responders achieved an ACR20 response by W52.
Conclusion: In this cohort of W24 ACR20 non-responders, GUS-treated pts experienced greater benefits than pts receiving PBO in improving both joint disease and other important PsA domains outside the ACR response criteria, which translated to significant improvements in health-related quality of life. These benefits occurred as early as W2 of GUS treatment and showed continued improvement over 24 W, such that considerable proportions of W24 ACR non-responders achieved an ACR20 response by W52. Such improvements in key PsA domains over time among the minority of GUS W24 non-responders is consistent with the known immunomodulatory mechanism of GUS.
Disclosures: D. McGonagle, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, UCB, Bristol-Myers Squibb(BMS), Amgen, Gilead, Pfizer; D. Haaland, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Janssen, Novartis, Pfizer, Roche, Sanofi Genzyme, Takeda, Merck/MSD, UCB, Celgene, Adiga Life-Sciences, Can-Fite Biopharma, Regeneron, Gilead; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis; A. Marrache, Janssen Pharmaceutical Companies of Johnson & Johnson, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; E. Rampakakis, Janssen, JSS Medical Research; P. Nash, AbbVie, Eli Lilly, Janssen, Gilead, Bristol-Myers Squibb (BMS), Celgene; F. Irazoque Palazuelos, AbbVie, Roche, Pfizer, Janssen; A. Kavanaugh, AbbVie, Amgen, Pfizer, Bristol-Myers Squibb(BMS), Centocor-Janssen, UCB, AstraZeneca, Roche.
Background/Purpose: Despite available PsA treatments, a portion of PsA patients (pts) does not achieve improvements in PsA signs and symptoms according to ACR response criteria. Using data from the phase 3 DISCOVER-1 and DISCOVER-2 (D1/D2) studies in pts with active PsA, the purpose of this analysis was to describe the benefit of the IL-23p19 inhibitor, guselkumab (GUS), vs placebo (PBO) across key PsA domains, including those not comprising the core ACR measures, in pts not meeting ACR response criteria.
Methods: Pts enrolled in D1 and D2 were adults with active PsA despite standard therapies. D1 pts had ≥3 swollen and ≥3 tender joints (SJC; TJC) and CRP ≥0.3 mg/dL; D2 pts had SJC ≥5, TJC ≥5 TJC, and CRP ≥0.6 mg/dL. 31% of D1 pts received 1-2 prior tumor necrosis factor inhibitors (TNFi); D2 pts were biologic-naïve. Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or PBO; PBO pts crossed over to GUS 100 mg Q4W at W24. In this post hoc analysis, patients not meeting ≥20% improvement in ACR response criteria (ACR20) at W24 were included. Changes from baseline (BL) over 24 W in continuous outcomes of interest (SJC; TJC; enthesitis/dactylitis scores; psoriasis area and severity index [PASI] score; Functional-Assessment of Chronic Illness Therapy [FACIT]-fatigue score; and SF-36 physical [PCS] & mental [MCS] component summary scores) were assessed with repeated measures mixed models adjusting for treatment group, baseline non-biologic DMARD use, and prior TNFi use. Descriptive statistics were produced for categorical outcomes at W24 (Figure 1) using non-responder imputation for missing data.
Results: Of the 1120 pooled D1/D2 pts, 538 did into achieve an ACR20 response at W24, including 137 (36.7%) GUS Q4W, 147 (39.2%) GUS Q8W, and 254 (68.3%) PBO pts, and were included in this analysis. A consistently greater proportion of GUS- than PBO-treated pts achieved W24 categorical outcomes, including those relating to skin, tender joints, and dactylitis, with similar findings for GUS Q4W and Q8W (Figure 1). For continuous endpoints, the benefit of GUS was seen as early as W2 for SJC (W4 for Q8W), TJC, dactylitis (W4 for Q8W), and enthesitis (W4 for Q8W); W8 for FACIT-fatigue, SF-36 PCS, and SF-36 MCS (W16 for Q8W); and W16 for PASI; most endpoints continued to improve through W24 (Figure 2). Across both GUS treatment groups, 43.5%-48.9% of W24 ACR20 non-responders achieved an ACR20 response by W52.
Conclusion: In this cohort of W24 ACR20 non-responders, GUS-treated pts experienced greater benefits than pts receiving PBO in improving both joint disease and other important PsA domains outside the ACR response criteria, which translated to significant improvements in health-related quality of life. These benefits occurred as early as W2 of GUS treatment and showed continued improvement over 24 W, such that considerable proportions of W24 ACR non-responders achieved an ACR20 response by W52. Such improvements in key PsA domains over time among the minority of GUS W24 non-responders is consistent with the known immunomodulatory mechanism of GUS.


Disclosures: D. McGonagle, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, UCB, Bristol-Myers Squibb(BMS), Amgen, Gilead, Pfizer; D. Haaland, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Janssen, Novartis, Pfizer, Roche, Sanofi Genzyme, Takeda, Merck/MSD, UCB, Celgene, Adiga Life-Sciences, Can-Fite Biopharma, Regeneron, Gilead; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis; A. Marrache, Janssen Pharmaceutical Companies of Johnson & Johnson, Johnson & Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; E. Rampakakis, Janssen, JSS Medical Research; P. Nash, AbbVie, Eli Lilly, Janssen, Gilead, Bristol-Myers Squibb (BMS), Celgene; F. Irazoque Palazuelos, AbbVie, Roche, Pfizer, Janssen; A. Kavanaugh, AbbVie, Amgen, Pfizer, Bristol-Myers Squibb(BMS), Centocor-Janssen, UCB, AstraZeneca, Roche.

