Back
Poster Session D
Immunobiology
Session: (1681–1706) Innate Immunity Poster: Basic and Translational Science
1683: Malondialdehyde-Acetaldehyde (MAA) Modified Matrix Gla Protein (MGP) Increases Citrullination by Human Macrophages
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SJ
Spencer Jones, BS
University of Nebraska College of Medicine
OMAHA, NE, United States
Abstract Poster Presenter(s)
Spencer Jones1, nozima Aripova1, Michael Duryee1, Austin Ragland1, Bryant England1, Ted Mikuls2 and Geoffrey Thiele1, 1University of Nebraska Medical Center, Omaha, NE, 2Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Malondialdehyde and acetaldehyde, by-products of lipid peroxidation, react with free amino groups on proteins to form a stable post-translational modification (PTM), termed MAA. MAA modifications and associated anti-MAA antibodies have been implicated in the pathogenesis of rheumatoid arthritis (RA). In RA synovium, MAA adducts have been shown to co-localize with another PTM, citrullination. Citrullination is mediated by the calcium-dependent enzyme peptidyl arginine deiminase (PAD), and its activity is dependent on tightly regulated intracellular calcium concentrations. One such intracellular calcium regulator is matrix gla protein (MGP), which functions as an intracellular calcium scavenger. The goal of this study was to evaluate potential changes in PAD expression and citrullination that may occur as a result of MAA modification of MGP.
Methods: A human monocytic cell line (U-937) was differentiated to a macrophage phenotype using phorbol 12-myristate 13-acetate (PMA), and subsequently stimulated for 6.5, 24, and 48 hours with either; native MGP or MAA-modified MGP (MGP-MAA). RT-PCR was performed for the following calcium modulators: inositol 1,4,5-Trisphosphate Receptor Interacting Protein (ITPRIPL), calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2), PAD2, and PAD4. Additionally, antigen-stimulated U-937 cells were lysed for protein purification and analyzed using Western Blotting for citrullinated (CIT) proteins using an anti-CIT antibody. The relative expression of protein quantity was normalized to β-actin (endogenous control).
Results: In comparison to native MGP, differentiated U-937 cells stimulated with MGP-MAA demonstrated an increase in mRNA expression for ITPRIPL (2.5-fold, p< 0.001) at 6.5 hours, PAD2 (3.4-fold, p< 0.001) at 48 hours, and PAD4 (5-fold, p< 0.001) at both 24 and 48h (Table 1). For Western Blot, MGP-MAA stimulated cells showed a 10-fold increase (p< 0.001) in citrullinated proteins compared to native MGP stimulated cells with the most prominent band identified at 75 kDA, which is suspected to be PAD2 based on preliminary data (Figure 1).
Conclusion: Although mechanisms underpinning these observations need to be elucidated, these studies suggest that MAA adduction of MGP likely diminishes its function as a calcium scavenger, resulting in an upregulation of key cellular calcium binding proteins. Amongst them is the calcium-dependent enzyme PAD2 and PAD4, which catalyzes the citrullination of proteins. The identification of a prominent 75 kDA band (a molecular weight corresponding to PAD) suggests the possibility that stimulation of cells with MAA-modified MGP may lead to the auto-citrullination of PAD. Taken together, these findings provide insight into a potential mechanism by which increased levels of citrullinated proteins in macrophages are produced resulting in the formation of autoantigens and autoantibodies unique to RA.
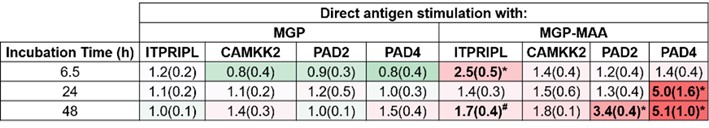 Table 1. RT-PCR from Stimulated U937 cells. U937 cells were stimulated with either native MGP or MAA adducted MGP (MGP-MAA). Cells were stimulated for 6.5, 24, or 48 hours before RNA collection and cDNA transformation. Primers for inositol 1,4,5-Trisphosphate Receptor Interacting Protein (ITPRIPL), calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2), PAD2, and PAD4 were utilized. All values from MGP-MAA stimulation were compared to MGP stimulation and expressed as relative quantification (RQ) (*p < 0.001, #p < 0.05).
Table 1. RT-PCR from Stimulated U937 cells. U937 cells were stimulated with either native MGP or MAA adducted MGP (MGP-MAA). Cells were stimulated for 6.5, 24, or 48 hours before RNA collection and cDNA transformation. Primers for inositol 1,4,5-Trisphosphate Receptor Interacting Protein (ITPRIPL), calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2), PAD2, and PAD4 were utilized. All values from MGP-MAA stimulation were compared to MGP stimulation and expressed as relative quantification (RQ) (*p < 0.001, #p < 0.05).
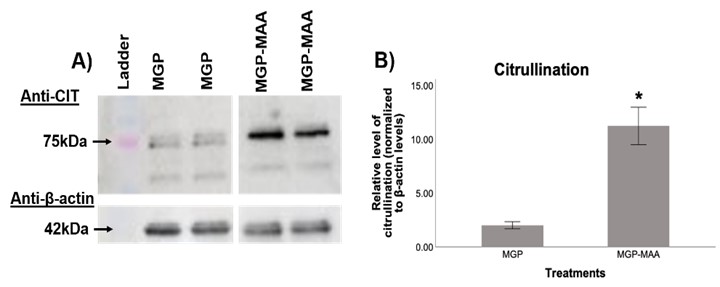 Figure 1. Western Blot from Stimulated U937 cells. U937 cells were stimulated with either native MGP or MAA adducted MGP (MGP-MAA). Cell lysates were probed with (A) anti-citrulline and anti-β-actin antibodies. Densitometry of normalized values to β-actin of (B) 75 kDa citrullination band for CIT, which is suspected to be PAD-2 based off preliminary data. All values from MGP-MAA stimulation were compared to MGP stimulation (*p < 0.001, #p < 0.05).
Figure 1. Western Blot from Stimulated U937 cells. U937 cells were stimulated with either native MGP or MAA adducted MGP (MGP-MAA). Cell lysates were probed with (A) anti-citrulline and anti-β-actin antibodies. Densitometry of normalized values to β-actin of (B) 75 kDa citrullination band for CIT, which is suspected to be PAD-2 based off preliminary data. All values from MGP-MAA stimulation were compared to MGP stimulation (*p < 0.001, #p < 0.05).
Disclosures: S. Jones, None; n. Aripova, None; M. Duryee, None; A. Ragland, None; B. England, Boehringer-Ingelheim; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; G. Thiele, None.
Background/Purpose: Malondialdehyde and acetaldehyde, by-products of lipid peroxidation, react with free amino groups on proteins to form a stable post-translational modification (PTM), termed MAA. MAA modifications and associated anti-MAA antibodies have been implicated in the pathogenesis of rheumatoid arthritis (RA). In RA synovium, MAA adducts have been shown to co-localize with another PTM, citrullination. Citrullination is mediated by the calcium-dependent enzyme peptidyl arginine deiminase (PAD), and its activity is dependent on tightly regulated intracellular calcium concentrations. One such intracellular calcium regulator is matrix gla protein (MGP), which functions as an intracellular calcium scavenger. The goal of this study was to evaluate potential changes in PAD expression and citrullination that may occur as a result of MAA modification of MGP.
Methods: A human monocytic cell line (U-937) was differentiated to a macrophage phenotype using phorbol 12-myristate 13-acetate (PMA), and subsequently stimulated for 6.5, 24, and 48 hours with either; native MGP or MAA-modified MGP (MGP-MAA). RT-PCR was performed for the following calcium modulators: inositol 1,4,5-Trisphosphate Receptor Interacting Protein (ITPRIPL), calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2), PAD2, and PAD4. Additionally, antigen-stimulated U-937 cells were lysed for protein purification and analyzed using Western Blotting for citrullinated (CIT) proteins using an anti-CIT antibody. The relative expression of protein quantity was normalized to β-actin (endogenous control).
Results: In comparison to native MGP, differentiated U-937 cells stimulated with MGP-MAA demonstrated an increase in mRNA expression for ITPRIPL (2.5-fold, p< 0.001) at 6.5 hours, PAD2 (3.4-fold, p< 0.001) at 48 hours, and PAD4 (5-fold, p< 0.001) at both 24 and 48h (Table 1). For Western Blot, MGP-MAA stimulated cells showed a 10-fold increase (p< 0.001) in citrullinated proteins compared to native MGP stimulated cells with the most prominent band identified at 75 kDA, which is suspected to be PAD2 based on preliminary data (Figure 1).
Conclusion: Although mechanisms underpinning these observations need to be elucidated, these studies suggest that MAA adduction of MGP likely diminishes its function as a calcium scavenger, resulting in an upregulation of key cellular calcium binding proteins. Amongst them is the calcium-dependent enzyme PAD2 and PAD4, which catalyzes the citrullination of proteins. The identification of a prominent 75 kDA band (a molecular weight corresponding to PAD) suggests the possibility that stimulation of cells with MAA-modified MGP may lead to the auto-citrullination of PAD. Taken together, these findings provide insight into a potential mechanism by which increased levels of citrullinated proteins in macrophages are produced resulting in the formation of autoantigens and autoantibodies unique to RA.
 Table 1. RT-PCR from Stimulated U937 cells. U937 cells were stimulated with either native MGP or MAA adducted MGP (MGP-MAA). Cells were stimulated for 6.5, 24, or 48 hours before RNA collection and cDNA transformation. Primers for inositol 1,4,5-Trisphosphate Receptor Interacting Protein (ITPRIPL), calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2), PAD2, and PAD4 were utilized. All values from MGP-MAA stimulation were compared to MGP stimulation and expressed as relative quantification (RQ) (*p < 0.001, #p < 0.05).
Table 1. RT-PCR from Stimulated U937 cells. U937 cells were stimulated with either native MGP or MAA adducted MGP (MGP-MAA). Cells were stimulated for 6.5, 24, or 48 hours before RNA collection and cDNA transformation. Primers for inositol 1,4,5-Trisphosphate Receptor Interacting Protein (ITPRIPL), calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2), PAD2, and PAD4 were utilized. All values from MGP-MAA stimulation were compared to MGP stimulation and expressed as relative quantification (RQ) (*p < 0.001, #p < 0.05). Figure 1. Western Blot from Stimulated U937 cells. U937 cells were stimulated with either native MGP or MAA adducted MGP (MGP-MAA). Cell lysates were probed with (A) anti-citrulline and anti-β-actin antibodies. Densitometry of normalized values to β-actin of (B) 75 kDa citrullination band for CIT, which is suspected to be PAD-2 based off preliminary data. All values from MGP-MAA stimulation were compared to MGP stimulation (*p < 0.001, #p < 0.05).
Figure 1. Western Blot from Stimulated U937 cells. U937 cells were stimulated with either native MGP or MAA adducted MGP (MGP-MAA). Cell lysates were probed with (A) anti-citrulline and anti-β-actin antibodies. Densitometry of normalized values to β-actin of (B) 75 kDa citrullination band for CIT, which is suspected to be PAD-2 based off preliminary data. All values from MGP-MAA stimulation were compared to MGP stimulation (*p < 0.001, #p < 0.05).Disclosures: S. Jones, None; n. Aripova, None; M. Duryee, None; A. Ragland, None; B. England, Boehringer-Ingelheim; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc; G. Thiele, None.

