Back
Abstract Session
Epidemiology, health policy and outcomes
Session: Abstracts: Measures and Measurement of Healthcare Quality (0570–0573)
0572: ACR Workgroup to Develop Recommendations for PRO Use in Clinical Care for SLE
Sunday, November 13, 2022
8:30 AM – 8:40 AM Eastern Time
Location: Room 121
.jpg)
Patricia Katz, PhD
UCSF
San Rafael, CA, United States
Presenting Author(s)
Patricia Katz1, Claire Barber2, Ali Duarte-Garcia3, Shivani Garg4, Wambui Machua5, Yesenia Santiago-Casas6, Wendy Rodgers7, Lisa Suter8, Jennifer Ude9, Tracy Johansson9, Christie Bartels10 and Jinoos Yazdany11, 1UCSF, San Rafael, CA, 2University of Calgary, Calgary, AB, Canada, 3Mayo Clinic, Rochester, MN, 4University of Madison, School of Medicine and Public Health, Madison, WI, 5Piedmont Physicians, Rheumatology, Atlanta, GA, 6Integral Rheumatology and Immunology Specialists, Plantation, FL, 7Lupus Foundation of America, Torrance, CA, 8Yale School of Medicine, New Haven, CT, 9American College of Rheumatology, Atlanta, GA, 10University of Wisconsin School of Medicine and Public Health, Madison, WI, 11UCSF, San Francisco, CA
Background/Purpose: Patient-reported outcome (PRO) measures are not consistently used in clinical settings for patients with systemic lupus erythematosus (SLE). As part of a Centers for Disease Control and Prevention-funded ACR initiative to develop RISE registry digital quality measures for SLE, we sought to develop recommendations for the content of a quality measure related to PRO use in SLE clinical care.
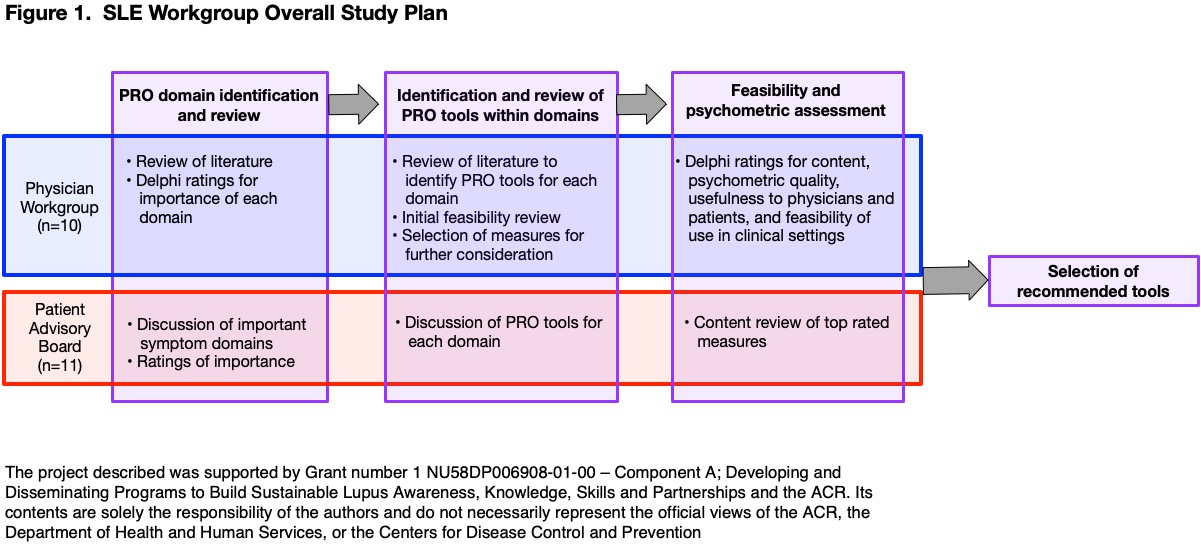
Methods: Figure 1 illustrates the overall study plan. First, an expert panel composed of physician, patient, and researcher representatives conducted a structured literature search to identify PRO domains of greatest importance to people with SLE. Papers that reported the patient's perspective on important patient-reported domains were identified. Data were extracted from all included studies. In a modified Delphi process, the perceived importance of identified domains was ranked. A patient focus group was also convened to identify and rank the importance of PRO domains. After identifying domains, PRO measures (PROMs) assessing each domain were identified through a structured literature review. Detailed evaluations, including psychometric reviews of use in general settings and specifically in SLE, were conducted for each PROM. In a second Delphi process, the expert panel rated PROMs on content, psychometric quality, and feasibility for use in routine SLE clinical care.
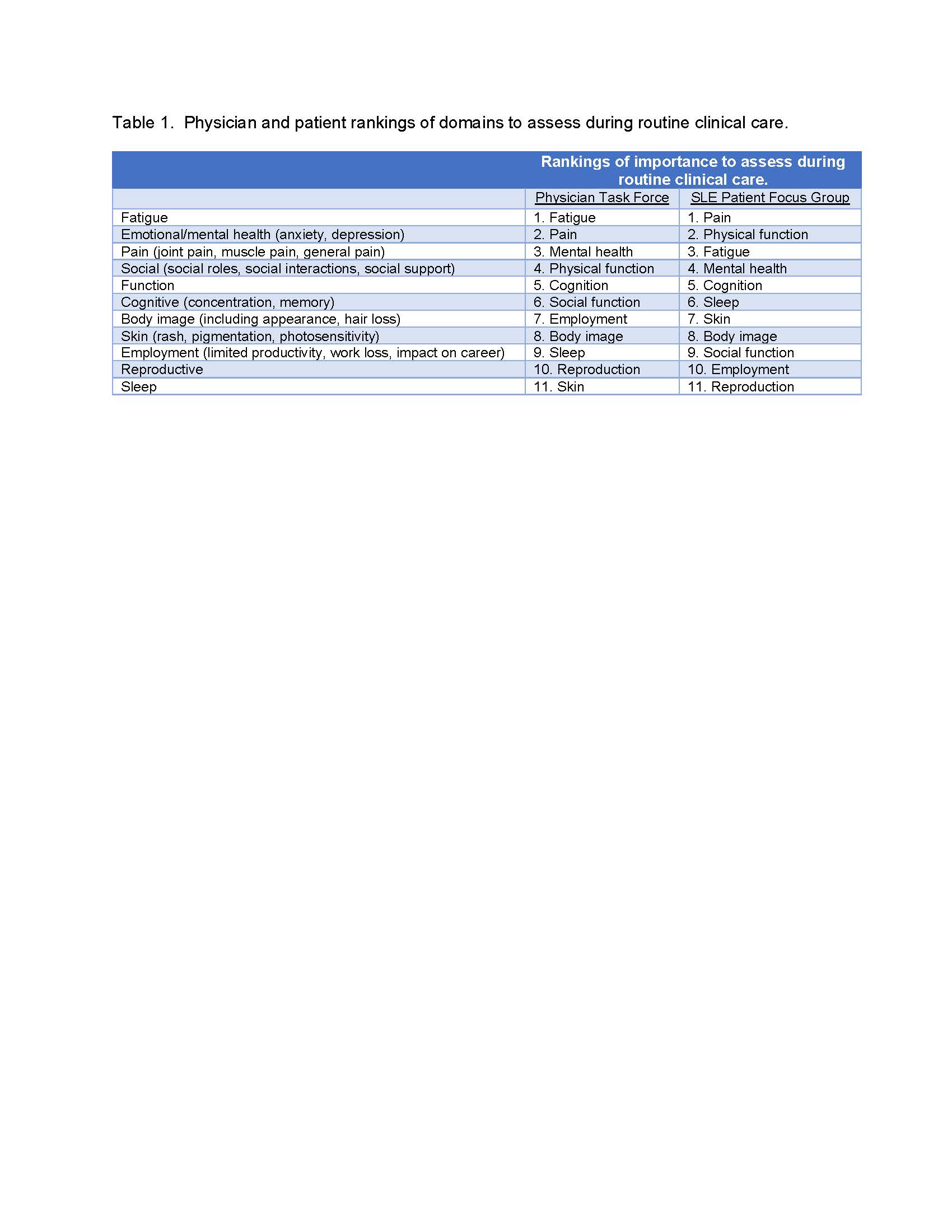
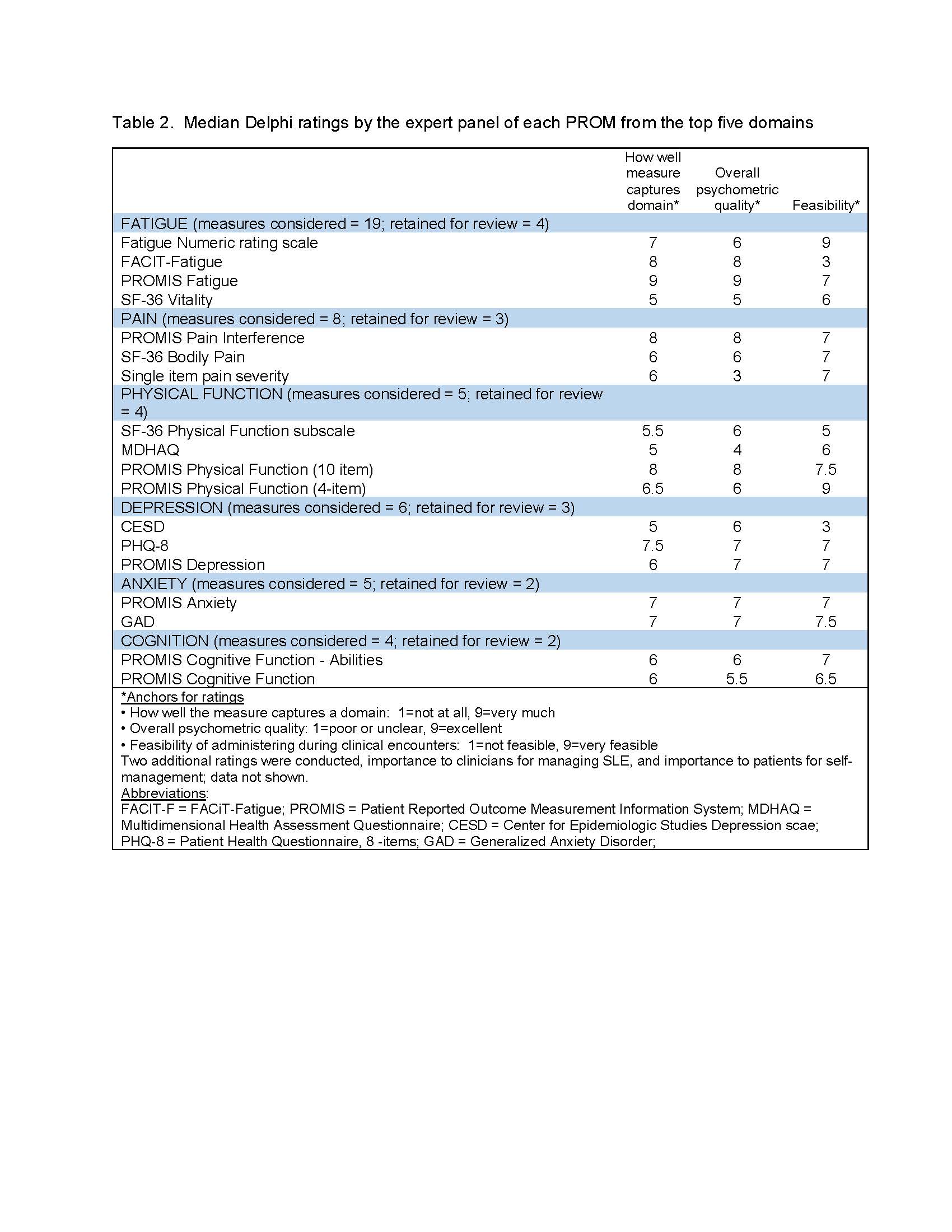
Results: In the structured literature search, 2505 papers were identified. Additional titles were identified by experts or through manual searches. After review of titles and abstracts, 56 papers identifying important PRO domains in SLE were reviewed. Data extracted from each paper included information on identification of patient outcomes or priority of outcomes. 27 papers yielded relevant content. The most frequently mentioned domains were fatigue, pain, social functioning, physical functioning, body image and concerns, and cognition, each of which was mentioned in at least 50% of the papers. The Delphi exercise with expert panelists and the focus group with patients both ranked fatigue, pain, mental health (e.g. depression and anxiety), physical function, and cognition as the top 5 domains for assessment during routine SLE clinical care (Table 1). 47 PROMs were mapped to these domains. Some PROMs were removed from consideration because they had not been used in SLE or had not been used in the past 10 years, required fees for use, or were not available in languages other than English. PROMs retained for each top domain and median ratings by the expert panel for content, psychometric quality, and feasibility of use in clinical settings are shown in Table 2.
Conclusion: Physicians and people with SLE identified 5 key areas for PRO measurement in clinical settings: fatigue, pain, mental health, physical function, and cognition. Measurement tools that were rated highly in terms of content, quality, and feasibility were identified for each domain. Next steps are to develop recommendations for PROs to collect routinely in SLE clinical care, and to develop a quality measure that incorporates this PRO measurement strategy into the RISE registry.
Disclosures: P. Katz, None; C. Barber, None; A. Duarte-Garcia, None; S. Garg, None; W. Machua, None; Y. Santiago-Casas, None; W. Rodgers, None; L. Suter, None; J. Ude, None; T. Johansson, None; C. Bartels, Pfizer; J. Yazdany, AstraZeneca, Gilead, Bristol-Myers Squibb(BMS), Aurinia, Astra Zeneca, Pfizer.
Background/Purpose: Patient-reported outcome (PRO) measures are not consistently used in clinical settings for patients with systemic lupus erythematosus (SLE). As part of a Centers for Disease Control and Prevention-funded ACR initiative to develop RISE registry digital quality measures for SLE, we sought to develop recommendations for the content of a quality measure related to PRO use in SLE clinical care.
Methods: Figure 1 illustrates the overall study plan. First, an expert panel composed of physician, patient, and researcher representatives conducted a structured literature search to identify PRO domains of greatest importance to people with SLE. Papers that reported the patient's perspective on important patient-reported domains were identified. Data were extracted from all included studies. In a modified Delphi process, the perceived importance of identified domains was ranked. A patient focus group was also convened to identify and rank the importance of PRO domains. After identifying domains, PRO measures (PROMs) assessing each domain were identified through a structured literature review. Detailed evaluations, including psychometric reviews of use in general settings and specifically in SLE, were conducted for each PROM. In a second Delphi process, the expert panel rated PROMs on content, psychometric quality, and feasibility for use in routine SLE clinical care.
Results: In the structured literature search, 2505 papers were identified. Additional titles were identified by experts or through manual searches. After review of titles and abstracts, 56 papers identifying important PRO domains in SLE were reviewed. Data extracted from each paper included information on identification of patient outcomes or priority of outcomes. 27 papers yielded relevant content. The most frequently mentioned domains were fatigue, pain, social functioning, physical functioning, body image and concerns, and cognition, each of which was mentioned in at least 50% of the papers. The Delphi exercise with expert panelists and the focus group with patients both ranked fatigue, pain, mental health (e.g. depression and anxiety), physical function, and cognition as the top 5 domains for assessment during routine SLE clinical care (Table 1). 47 PROMs were mapped to these domains. Some PROMs were removed from consideration because they had not been used in SLE or had not been used in the past 10 years, required fees for use, or were not available in languages other than English. PROMs retained for each top domain and median ratings by the expert panel for content, psychometric quality, and feasibility of use in clinical settings are shown in Table 2.
Conclusion: Physicians and people with SLE identified 5 key areas for PRO measurement in clinical settings: fatigue, pain, mental health, physical function, and cognition. Measurement tools that were rated highly in terms of content, quality, and feasibility were identified for each domain. Next steps are to develop recommendations for PROs to collect routinely in SLE clinical care, and to develop a quality measure that incorporates this PRO measurement strategy into the RISE registry.



Disclosures: P. Katz, None; C. Barber, None; A. Duarte-Garcia, None; S. Garg, None; W. Machua, None; Y. Santiago-Casas, None; W. Rodgers, None; L. Suter, None; J. Ude, None; T. Johansson, None; C. Bartels, Pfizer; J. Yazdany, AstraZeneca, Gilead, Bristol-Myers Squibb(BMS), Aurinia, Astra Zeneca, Pfizer.

