Back
Poster Session D
Session: (1924–1949) Pediatric Rheumatology – Clinical Poster III: Other
1931: NOD2 Mutations Are Associated with Upregulated Type 1 Interferon Gene Expression and Development of Granulomatous Hepatitis in Children with Autoimmune Hepatitis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- EE
Esraa Eloseily, MD
Cincinnati Children's Hospital Medical Center
Cincinnati, OH, United States
Abstract Poster Presenter(s)
Esraa Eloseily1, Alexander Valencia2, Astha Malik2, Rebekah Karns2, Cyd Castro Rojas2, Mosab Alquraish2, Rebecca Marsh3, Alexei Grom4 and Alexander Miethke2, 1Division of Pediatric Rheumatology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 2Division of Gastroenterology, Hepatology & Nutrition, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 3Division of Bone Marrow Transplantation & Immune Deficiency, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 4Divisions of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
Background/Purpose: Mutations in the gene NOD2 encoding the nucleotide-binding oligomerization domain-containing protein 2 (NOD2) which controls innate responses to LPS have been linked to Blau syndrome or Crohn disease when affecting the NOD or LRR domains, respectively. The role of variants in NOD2 in autoimmune hepatitis (AIH) is unknown.
Methods: Patients with pediatric AIH or PSC were surveyed for variants in NOD2 using research whole exome/genome sequencing (n=55) or clinical NGS (n=8). Purified RNA from circulating leukocytes of patients (n=13, including 3 subject with variants in NOD2) and healthy controls ([HC]; n=6) was subjected to NanoString-based quantitation of expression of 52 genes associated with activation of type 1/2 interferons or NF-kB.
Results: Exome sequencing revealed three heterozygous variants in NOD2 in 5 patients, including the two risk alleles for Crohn disease, p.Gly908Arg and p.Leu1007Profs*2, and p.Asn289Ser, a variant of uncertain significance which localizes upstream of NOD (see table). Patient #1 presented with hepatitis and hypoxemia attributed to hepatopulmonary syndrome. Hepatitis improved with mycophenolate mofetil (MMF), corticosteroids (CS), and later tacrolimus (Tac), but repeat liver biopsy at age 16 showed active granulomatous hepatitis which was accompanied by elevated plasma level for CXCL9 of 3709 (normal< 121 pg/ml), a chemokine induced by IFNg. Patient #2 presented with severe hepatitis, and elevated plasma CXL9 of 9364 pg/ml. On treatment with MMF and CS, LFTs normalized but aplastic anemia developed. Patient #3 presented with hepatitis, Coombs+ hemolytic anemia, and was later diagnosed with SLE. Disease partially responded to MMF and CS. Subject #4 was diagnosed with celiac disease and AIH which was refractory to treatment with azathioprine (AZA), CS, and Tac. Case #5 had AIH which responded promptly to AZA and CS. No patient was diagnosed with IBD. NanoString-based quantitation of interferon and NF-kB gene activation revealed case 5 to segregate with HC in unsupervised analysis, while both subjects with the p.Gly908Arg mutation displayed upregulation of the genes CXCL10, EPSTI1, GBP1, IFIT2, and RTP4 compared with other AILD patients and HC.
Conclusion: NOD2 mutations affecting the LRR domain are associated with granulomatous hepatitis, extrahepatic immune dysregulation, and steroid refractory AIH. Whether upregulation of interferon responses in these patients requires alternative treatments needs further investigations.
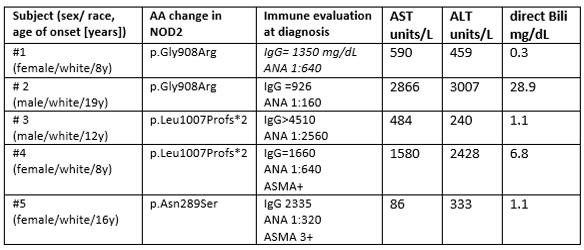 Demographics, genetic mutations, immune evaluation, liver enzymes and direct bilirubin levels of the 5 patients
Demographics, genetic mutations, immune evaluation, liver enzymes and direct bilirubin levels of the 5 patients
Disclosures: E. Eloseily, None; A. Valencia, None; A. Malik, None; R. Karns, None; C. Castro Rojas, None; M. Alquraish, None; R. Marsh, None; A. Grom, Sobi, Novartis; A. Miethke, None.
Background/Purpose: Mutations in the gene NOD2 encoding the nucleotide-binding oligomerization domain-containing protein 2 (NOD2) which controls innate responses to LPS have been linked to Blau syndrome or Crohn disease when affecting the NOD or LRR domains, respectively. The role of variants in NOD2 in autoimmune hepatitis (AIH) is unknown.
Methods: Patients with pediatric AIH or PSC were surveyed for variants in NOD2 using research whole exome/genome sequencing (n=55) or clinical NGS (n=8). Purified RNA from circulating leukocytes of patients (n=13, including 3 subject with variants in NOD2) and healthy controls ([HC]; n=6) was subjected to NanoString-based quantitation of expression of 52 genes associated with activation of type 1/2 interferons or NF-kB.
Results: Exome sequencing revealed three heterozygous variants in NOD2 in 5 patients, including the two risk alleles for Crohn disease, p.Gly908Arg and p.Leu1007Profs*2, and p.Asn289Ser, a variant of uncertain significance which localizes upstream of NOD (see table). Patient #1 presented with hepatitis and hypoxemia attributed to hepatopulmonary syndrome. Hepatitis improved with mycophenolate mofetil (MMF), corticosteroids (CS), and later tacrolimus (Tac), but repeat liver biopsy at age 16 showed active granulomatous hepatitis which was accompanied by elevated plasma level for CXCL9 of 3709 (normal< 121 pg/ml), a chemokine induced by IFNg. Patient #2 presented with severe hepatitis, and elevated plasma CXL9 of 9364 pg/ml. On treatment with MMF and CS, LFTs normalized but aplastic anemia developed. Patient #3 presented with hepatitis, Coombs+ hemolytic anemia, and was later diagnosed with SLE. Disease partially responded to MMF and CS. Subject #4 was diagnosed with celiac disease and AIH which was refractory to treatment with azathioprine (AZA), CS, and Tac. Case #5 had AIH which responded promptly to AZA and CS. No patient was diagnosed with IBD. NanoString-based quantitation of interferon and NF-kB gene activation revealed case 5 to segregate with HC in unsupervised analysis, while both subjects with the p.Gly908Arg mutation displayed upregulation of the genes CXCL10, EPSTI1, GBP1, IFIT2, and RTP4 compared with other AILD patients and HC.
Conclusion: NOD2 mutations affecting the LRR domain are associated with granulomatous hepatitis, extrahepatic immune dysregulation, and steroid refractory AIH. Whether upregulation of interferon responses in these patients requires alternative treatments needs further investigations.
 Demographics, genetic mutations, immune evaluation, liver enzymes and direct bilirubin levels of the 5 patients
Demographics, genetic mutations, immune evaluation, liver enzymes and direct bilirubin levels of the 5 patientsDisclosures: E. Eloseily, None; A. Valencia, None; A. Malik, None; R. Karns, None; C. Castro Rojas, None; M. Alquraish, None; R. Marsh, None; A. Grom, Sobi, Novartis; A. Miethke, None.

