Back
Poster Session D
Osteoarthritis (OA) and related disorders
Session: (1888–1923) Osteoarthritis – Clinical Poster
1891: Osteoarthritis of the Knee, Inflammation, and the Effect of Adalimumab: A Randomized Placebo-Controlled Trial
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- WM
Walter P. Maksymowych, MD
University of Alberta
Edmonton, AB, Canada
Abstract Poster Presenter(s)
Walter P Maksymowych1, Louis Bessette2, Robert G Lambert3, Amanda Carapellucci4 and Tom Appleton5, 1Department of Medicine, University of Alberta, Edmonton, AB, Canada, 2Centre de l'Ostoporose et de Rhumatologie de Québec, Québec, QC, Canada, 3University of Alberta, Edmonton, AB, Canada, 4CARE Arthritis LTD, Edmonton, AB, Canada, 5The University of Western Ontario, London, ON, Canada
Background/Purpose: Recent RCTs demonstrated that TNFα inhibition has no effect on pain and MRI-detected synovitis or bone marrow lesions in patients with erosive hand OA. However, the progression of bone erosions was reduced in a subgroup of patients with more clinically swollen distal interphalangeal joints in one RCT. Consequently, it remains possible that TNFα inhibition may have beneficial effects in specific subgroups of patients with a high inflammatory component. We aimed to evaluate the efficacy and safety of a TNFα inhibitor, adalimumab (ADA), in a proof-of-concept study in patients with inflammatory OA of the knee.
Methods: OKINADA was a 52-week, randomized, double-blind, placebo-controlled, parallel-group study done at 11 sites in Canada (NCT02471118). Eligible participants were adults with a diagnosis of OA of the index knee and classified according to American College of Rheumatology criteria, including radiological evidence of OA (Kellgren-Lawrence grades 2 or 3), with clinical signs of knee effusion. Subjects had persistent knee pain of ≥ one month duration with a pain score of ≥ 4 (0-10 NRS) in the index knee at screening and baseline despite conventional treatment with maximum tolerated acetaminophen and/or non-steroidal anti-inflammatory drug. Patients were randomly assigned (1:1) to receive subcutaneous 40 mg ADA every 2 weeks or placebo (PBO) to week 16. All patients then received open label ADA to week 32. Primary endpoint was the Outcome Measures in Rheumatology and Osteoarthritis Research Society International set of responder criteria (OMERACT-OARSI) at week 16. Secondary endpoints included: the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the domains of pain, activities of daily living (ADL), OA symptoms, sport and recreation function (SRF), and knee-related quality of life (QoL), patient global assessment of disease status (PGAD), investigator global assessment of disease status (IGAD), and expanded Target Joint Assessment (TJA) score.
Results: A total of 58 patients were randomized (28 to PBO, 30 to ADA) and patients were well-matched for baseline characteristics (Table). The primary endpoint was not met: OMERACT-OARSI combined (ADA: 9 [30.0%] vs PBO: 7 [25%], p=0.90) (Figure 1). For KOOS pain, ≥20% improvement was noted in 11 (36.7%) ADA vs 7 (25.0%) PBO patients (p=0.50), and ≥50% improvement in 5 (16.7%) ADA vs 6 (21.4%) PBO patients (p=0.90). There were no significant treatment-group differences in baseline to 16-week change in continuous secondary endpoints (KOOS ADL-QoL-Symptoms-SRF, PGAD, IGAD, TJA, physical assessment of the target knee) or in lab markers (ESR, CRP) (Figure 2). OMERACT-OARSI response increased to 53.3% (ITT) at week 32 in the group randomized to ADA. There were 9 withdrawals (4 ADA, 5 PBO) to week 16 and 3 withdrawals between weeks 16-32 but no new safety signals were identified and there were no serious adverse events.
Conclusion: Although the treatment was safe, short-term treatment with anti-TNFα therapy does not appear to provide clinically meaningful improvements in OA symptoms though it remains possible that longer duration of treatment could be beneficial. Analyses of structural endpoints will be reported when results are available.
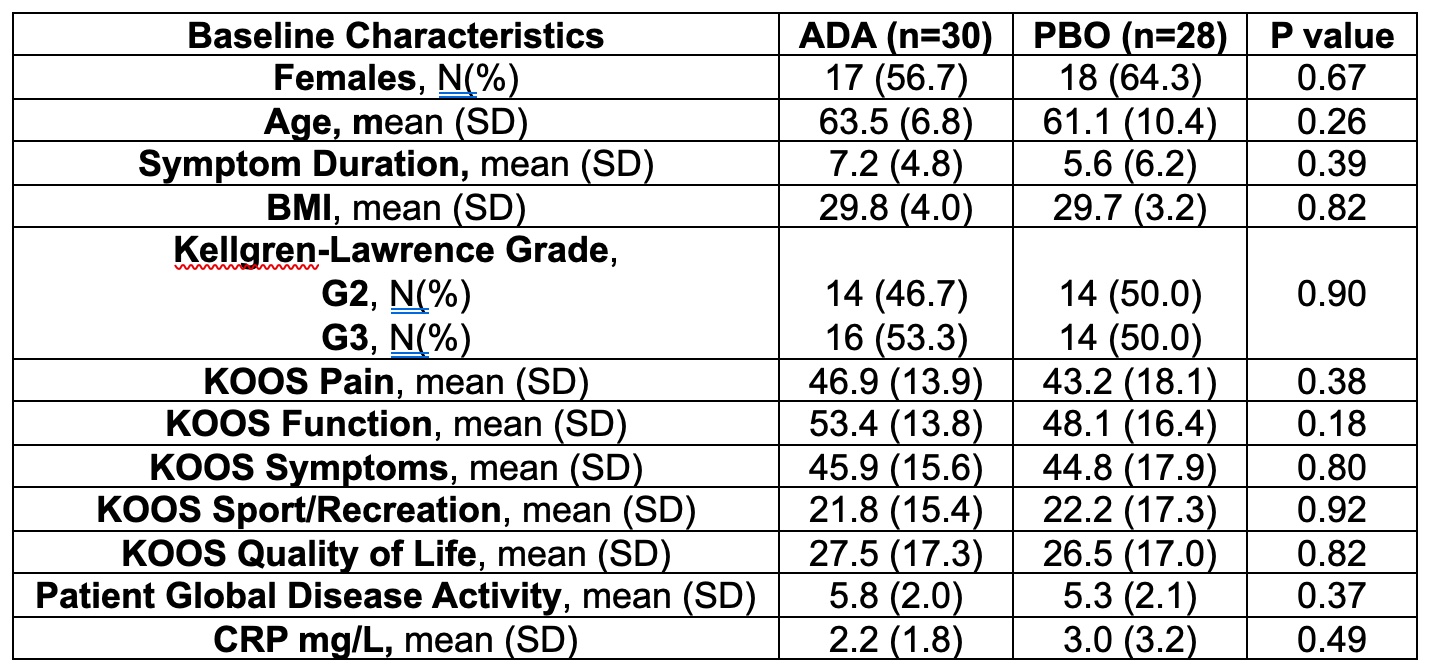 Table. Baseline characteristics of patients randomized 1:1 to either adalimumab or placebo.
Table. Baseline characteristics of patients randomized 1:1 to either adalimumab or placebo.
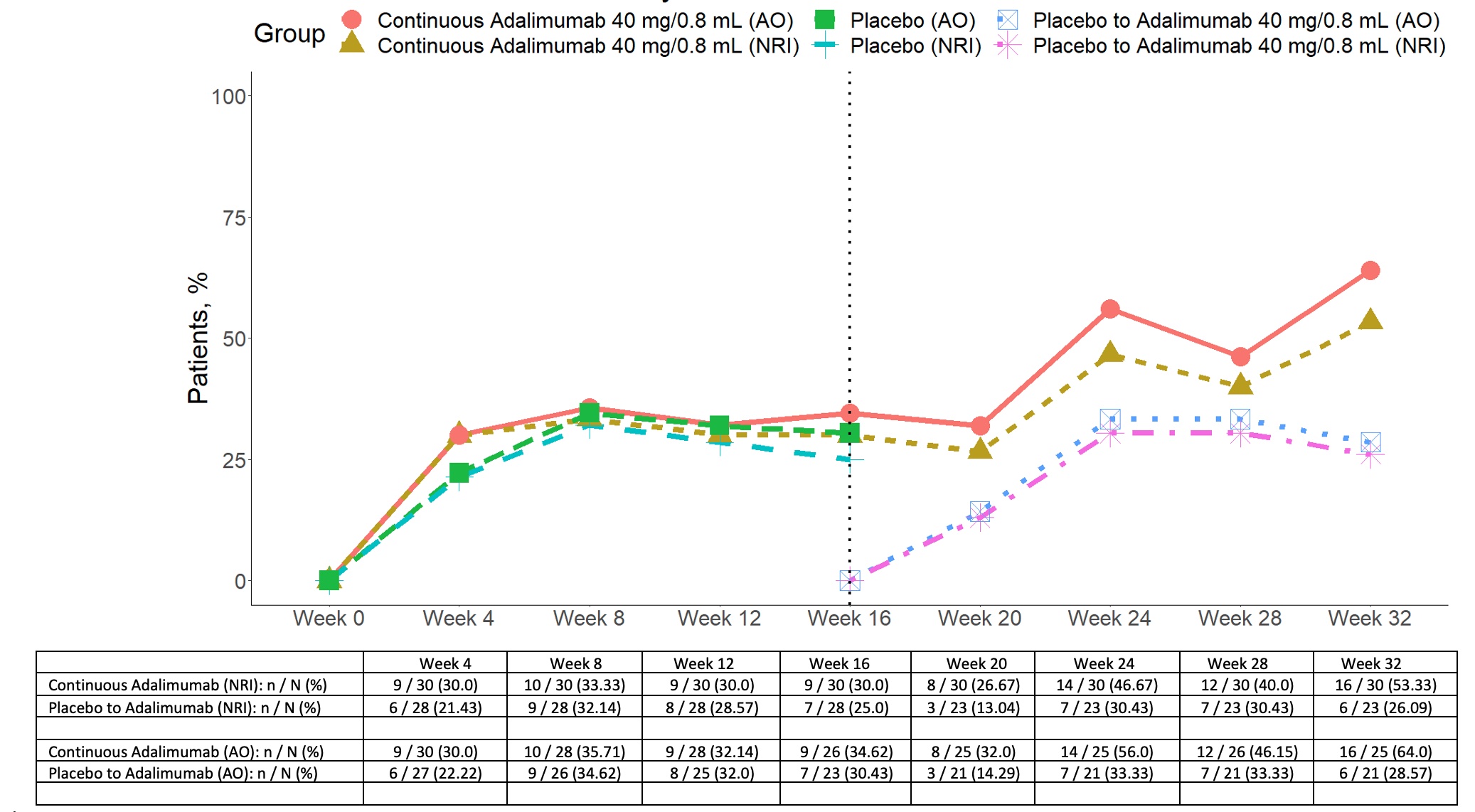 Figure 1. OMERACT-OARSI responses in patients with inflammatory knee osteoarthritis randomized 1:1 to adalimumab or placebo to week 16 and then all patients receiving open label adalimumab from 16-32 weeks. NRI non-responder imputation data AO as observed data
Figure 1. OMERACT-OARSI responses in patients with inflammatory knee osteoarthritis randomized 1:1 to adalimumab or placebo to week 16 and then all patients receiving open label adalimumab from 16-32 weeks. NRI non-responder imputation data AO as observed data
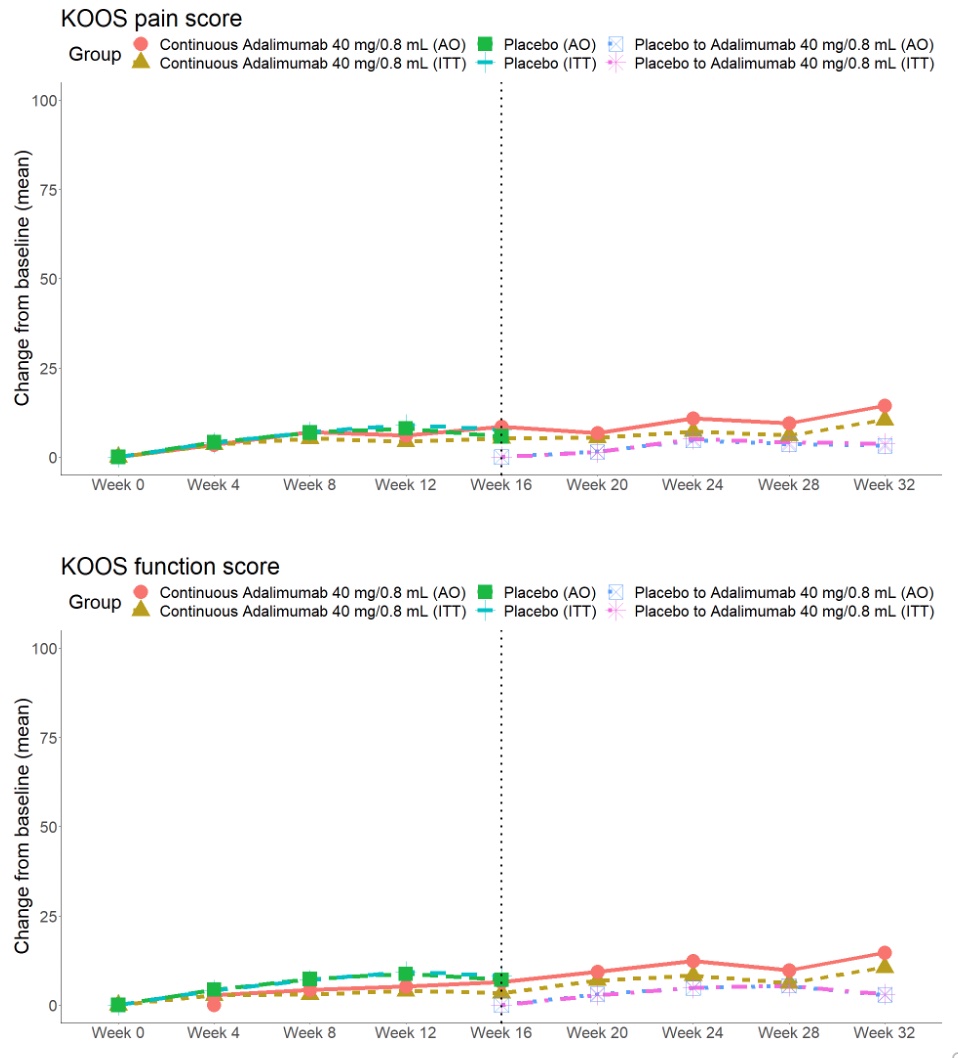 Figure 2. Change in KOOS Pain and Function in patients with knee osteoarthritis randomized 1:1 to adalimumab or placebo to 16 weeks and then all patients receiving open label adalimumab from 16-32 weeks.
Figure 2. Change in KOOS Pain and Function in patients with knee osteoarthritis randomized 1:1 to adalimumab or placebo to 16 weeks and then all patients receiving open label adalimumab from 16-32 weeks.
Disclosures: W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; R. Lambert, Calyx, CARE Arthritis, Image Analysis Group; A. Carapellucci, None; T. Appleton, AbbVie/Abbott, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Celgene, Fresenius Kabi, Gilead, Janssen, Merck/MSD, Novartis, Organon, Pfizer, Roche, UCB, Sanofi-Genzyme.
Background/Purpose: Recent RCTs demonstrated that TNFα inhibition has no effect on pain and MRI-detected synovitis or bone marrow lesions in patients with erosive hand OA. However, the progression of bone erosions was reduced in a subgroup of patients with more clinically swollen distal interphalangeal joints in one RCT. Consequently, it remains possible that TNFα inhibition may have beneficial effects in specific subgroups of patients with a high inflammatory component. We aimed to evaluate the efficacy and safety of a TNFα inhibitor, adalimumab (ADA), in a proof-of-concept study in patients with inflammatory OA of the knee.
Methods: OKINADA was a 52-week, randomized, double-blind, placebo-controlled, parallel-group study done at 11 sites in Canada (NCT02471118). Eligible participants were adults with a diagnosis of OA of the index knee and classified according to American College of Rheumatology criteria, including radiological evidence of OA (Kellgren-Lawrence grades 2 or 3), with clinical signs of knee effusion. Subjects had persistent knee pain of ≥ one month duration with a pain score of ≥ 4 (0-10 NRS) in the index knee at screening and baseline despite conventional treatment with maximum tolerated acetaminophen and/or non-steroidal anti-inflammatory drug. Patients were randomly assigned (1:1) to receive subcutaneous 40 mg ADA every 2 weeks or placebo (PBO) to week 16. All patients then received open label ADA to week 32. Primary endpoint was the Outcome Measures in Rheumatology and Osteoarthritis Research Society International set of responder criteria (OMERACT-OARSI) at week 16. Secondary endpoints included: the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the domains of pain, activities of daily living (ADL), OA symptoms, sport and recreation function (SRF), and knee-related quality of life (QoL), patient global assessment of disease status (PGAD), investigator global assessment of disease status (IGAD), and expanded Target Joint Assessment (TJA) score.
Results: A total of 58 patients were randomized (28 to PBO, 30 to ADA) and patients were well-matched for baseline characteristics (Table). The primary endpoint was not met: OMERACT-OARSI combined (ADA: 9 [30.0%] vs PBO: 7 [25%], p=0.90) (Figure 1). For KOOS pain, ≥20% improvement was noted in 11 (36.7%) ADA vs 7 (25.0%) PBO patients (p=0.50), and ≥50% improvement in 5 (16.7%) ADA vs 6 (21.4%) PBO patients (p=0.90). There were no significant treatment-group differences in baseline to 16-week change in continuous secondary endpoints (KOOS ADL-QoL-Symptoms-SRF, PGAD, IGAD, TJA, physical assessment of the target knee) or in lab markers (ESR, CRP) (Figure 2). OMERACT-OARSI response increased to 53.3% (ITT) at week 32 in the group randomized to ADA. There were 9 withdrawals (4 ADA, 5 PBO) to week 16 and 3 withdrawals between weeks 16-32 but no new safety signals were identified and there were no serious adverse events.
Conclusion: Although the treatment was safe, short-term treatment with anti-TNFα therapy does not appear to provide clinically meaningful improvements in OA symptoms though it remains possible that longer duration of treatment could be beneficial. Analyses of structural endpoints will be reported when results are available.
 Table. Baseline characteristics of patients randomized 1:1 to either adalimumab or placebo.
Table. Baseline characteristics of patients randomized 1:1 to either adalimumab or placebo. Figure 1. OMERACT-OARSI responses in patients with inflammatory knee osteoarthritis randomized 1:1 to adalimumab or placebo to week 16 and then all patients receiving open label adalimumab from 16-32 weeks. NRI non-responder imputation data AO as observed data
Figure 1. OMERACT-OARSI responses in patients with inflammatory knee osteoarthritis randomized 1:1 to adalimumab or placebo to week 16 and then all patients receiving open label adalimumab from 16-32 weeks. NRI non-responder imputation data AO as observed data Figure 2. Change in KOOS Pain and Function in patients with knee osteoarthritis randomized 1:1 to adalimumab or placebo to 16 weeks and then all patients receiving open label adalimumab from 16-32 weeks.
Figure 2. Change in KOOS Pain and Function in patients with knee osteoarthritis randomized 1:1 to adalimumab or placebo to 16 weeks and then all patients receiving open label adalimumab from 16-32 weeks.Disclosures: W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; R. Lambert, Calyx, CARE Arthritis, Image Analysis Group; A. Carapellucci, None; T. Appleton, AbbVie/Abbott, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Celgene, Fresenius Kabi, Gilead, Janssen, Merck/MSD, Novartis, Organon, Pfizer, Roche, UCB, Sanofi-Genzyme.

