Back
Poster Session D
Session: (1980–2016) RA – Treatment Poster IV
1999: Patient-Reported Outcomes in Rheumatoid Arthritis Correlate with Clinical Disease Activity Index Response in the Study to Accelerate Information of Molecular Signatures (AIMS)
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Vibeke Strand, MD, FACP, MACR
Adjunct Clinical Professor
Stanford University School of Medicine, Palo Alto CA
Portola Valley, CA, United States
Abstract Poster Presenter(s)
Vibeke Strand1, Emelly Rusli2, Lixia Zhang2, Christina Le-Short2, Alix Arnaud2, Johanna Withers3 and Sam Asgarian2, 1Stanford University School of Medicine, Stanford, CA, 2Scipher Medicine Corporation, Waltham, MA, 3Scipher Medicine Corportaion, Waltham, MA
Background/Purpose: Rheumatoid arthritis (RA) treatment response is typically defined using clinician-reported scores and patient global assessments of disease activity (PtGA), but patients may have a broader conceptualization of outcome, including physical function (Health Assessment Questionnaire Disability Index: HAQ-DI), pain, and fatigue levels. Patient-reported outcomes (PRO) play a crucial role that not only compliments clinical measures but also demonstrates treatment-associated improvements in broader health status. This study explored the association between Clinical Disease Activity Index (CDAI) responses to low disease activity (LDA) and remission (REM) and improvements in PtGA, pain, and HAQ-DI in patients with moderate-to-severely active RA.
Methods: Data from the Study to Accelerate Information of Molecular Signatures (AIMS), a clinical study of real-world longitudinal data from patients with RA managed across a network of private and academic rheumatology practices in the contiguous US were analyzed. Patient management in AIMS focuses on the utilization of a commercially available MSRC test. Adjusted mean improvements in CDAI, PtGA, pain, and HAQ-DI from baseline to 6 months and percentages reporting scores ≥ minimum clinically important differences (MCID, defined as improvement in PtGA and pain VAS ≥10 and HAQ-DI ≥0.22) were compared. Generalized linear modeling was employed to assess the associations between PROs with CDAI responses adjusted for baseline covariates such as age, gender, baseline CDAI, tender and swollen joint counts, PtGA, and HAQ-DI.
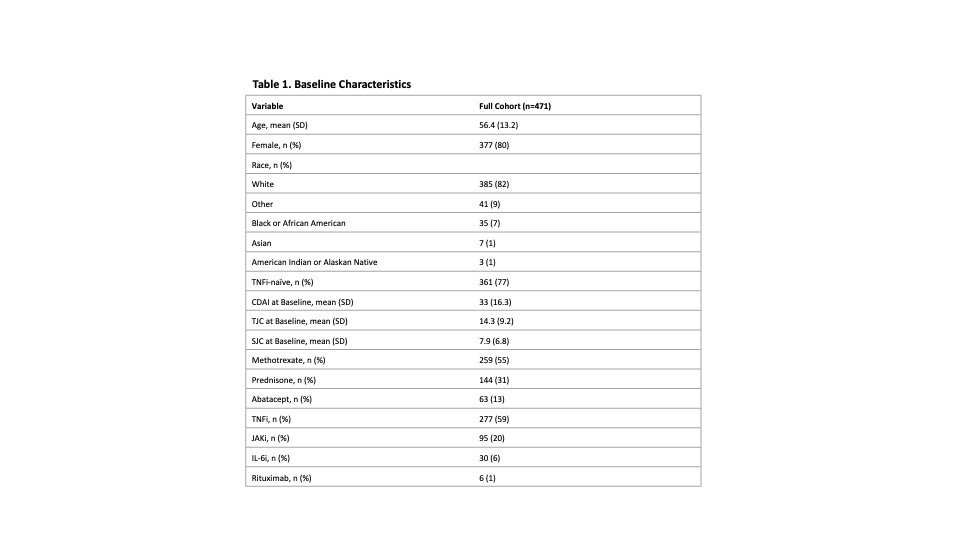
Results: A total of 471 patients were included; the mean age was 56.4 (SD=13.2) years, the mean baseline CDAI was 33 (SD= 16.3), 80% were female and 77% were TNFi-naïve (Table 1). At 6 months, improvements in all PROs in responders were statistically significant. More patients achieving CDAI-LDA and REM reported improvements in PtGA ≥ MCID (70% and 93%, respectively) than non-responders (47%, Table 2). PtGA, pain, and HAQ-DI improvements from baseline were significantly associated with CDAI-LDA responses (p<0.0001, Table 2). Specific distributions of mean PRO improvements by CDAI-LDA and REM status are shown in Figure 1.
Conclusion: RA patients who achieved CDAI-LDA or REM reported lower impacts of disease, less pain, and improved physical function. This study further underscores the importance of helping RA patients select the most effective therapy to reach treat-to-target goals, an area where precision medicine can fill a large unmet need.
.jpg)
.jpg) Figure 1. PRO association with CDAI response status. Left panels: Boxplots showing distributions of PRO (PtGA, pain score, HAQ-DI) score improvement according to CDAI status at 6 months. Sample size and least squares mean (lsmean) are reported. Right panels: Bar charts showing percentage of patients reporting PRO (PtGA, pain, HAQ-DI) score improvement ≥ clinically critical values according to CDAI status at 6 months, where critical values are defined as 10, 10, and 0.22 for PtGA, pain score, and HAQ-DI, respectively.
Figure 1. PRO association with CDAI response status. Left panels: Boxplots showing distributions of PRO (PtGA, pain score, HAQ-DI) score improvement according to CDAI status at 6 months. Sample size and least squares mean (lsmean) are reported. Right panels: Bar charts showing percentage of patients reporting PRO (PtGA, pain, HAQ-DI) score improvement ≥ clinically critical values according to CDAI status at 6 months, where critical values are defined as 10, 10, and 0.22 for PtGA, pain score, and HAQ-DI, respectively.
Disclosures: V. Strand, AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb(BMS), Boehringer-Ingelheim, Chemocentryx, Celltrion, Genentech/Roche, Gilead, GlaxoSmithKlein(GSK), Inmedix, Janssen, Kiniksa, Merck/MSD, Novartis, Pfizer, Regeneron Pharmaceuticals, Rheos, R-Pharma, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Spherix, Aria, Bioventus, Blackrock, Equilium, Glenmark, Horizon, Kypha, Lilly, MiMedx, Sorrento, Tonix, Priovant; E. Rusli, Scipher Medicine; L. Zhang, Scipher Medicine; C. Le-Short, Scipher Medicine Corporation; A. Arnaud, Scipher Medicine; J. Withers, Scipher Medicine Corporation; S. Asgarian, Scipher Medicine.
Background/Purpose: Rheumatoid arthritis (RA) treatment response is typically defined using clinician-reported scores and patient global assessments of disease activity (PtGA), but patients may have a broader conceptualization of outcome, including physical function (Health Assessment Questionnaire Disability Index: HAQ-DI), pain, and fatigue levels. Patient-reported outcomes (PRO) play a crucial role that not only compliments clinical measures but also demonstrates treatment-associated improvements in broader health status. This study explored the association between Clinical Disease Activity Index (CDAI) responses to low disease activity (LDA) and remission (REM) and improvements in PtGA, pain, and HAQ-DI in patients with moderate-to-severely active RA.
Methods: Data from the Study to Accelerate Information of Molecular Signatures (AIMS), a clinical study of real-world longitudinal data from patients with RA managed across a network of private and academic rheumatology practices in the contiguous US were analyzed. Patient management in AIMS focuses on the utilization of a commercially available MSRC test. Adjusted mean improvements in CDAI, PtGA, pain, and HAQ-DI from baseline to 6 months and percentages reporting scores ≥ minimum clinically important differences (MCID, defined as improvement in PtGA and pain VAS ≥10 and HAQ-DI ≥0.22) were compared. Generalized linear modeling was employed to assess the associations between PROs with CDAI responses adjusted for baseline covariates such as age, gender, baseline CDAI, tender and swollen joint counts, PtGA, and HAQ-DI.
Results: A total of 471 patients were included; the mean age was 56.4 (SD=13.2) years, the mean baseline CDAI was 33 (SD= 16.3), 80% were female and 77% were TNFi-naïve (Table 1). At 6 months, improvements in all PROs in responders were statistically significant. More patients achieving CDAI-LDA and REM reported improvements in PtGA ≥ MCID (70% and 93%, respectively) than non-responders (47%, Table 2). PtGA, pain, and HAQ-DI improvements from baseline were significantly associated with CDAI-LDA responses (p<0.0001, Table 2). Specific distributions of mean PRO improvements by CDAI-LDA and REM status are shown in Figure 1.
Conclusion: RA patients who achieved CDAI-LDA or REM reported lower impacts of disease, less pain, and improved physical function. This study further underscores the importance of helping RA patients select the most effective therapy to reach treat-to-target goals, an area where precision medicine can fill a large unmet need.

.jpg)
.jpg) Figure 1. PRO association with CDAI response status. Left panels: Boxplots showing distributions of PRO (PtGA, pain score, HAQ-DI) score improvement according to CDAI status at 6 months. Sample size and least squares mean (lsmean) are reported. Right panels: Bar charts showing percentage of patients reporting PRO (PtGA, pain, HAQ-DI) score improvement ≥ clinically critical values according to CDAI status at 6 months, where critical values are defined as 10, 10, and 0.22 for PtGA, pain score, and HAQ-DI, respectively.
Figure 1. PRO association with CDAI response status. Left panels: Boxplots showing distributions of PRO (PtGA, pain score, HAQ-DI) score improvement according to CDAI status at 6 months. Sample size and least squares mean (lsmean) are reported. Right panels: Bar charts showing percentage of patients reporting PRO (PtGA, pain, HAQ-DI) score improvement ≥ clinically critical values according to CDAI status at 6 months, where critical values are defined as 10, 10, and 0.22 for PtGA, pain score, and HAQ-DI, respectively. Disclosures: V. Strand, AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb(BMS), Boehringer-Ingelheim, Chemocentryx, Celltrion, Genentech/Roche, Gilead, GlaxoSmithKlein(GSK), Inmedix, Janssen, Kiniksa, Merck/MSD, Novartis, Pfizer, Regeneron Pharmaceuticals, Rheos, R-Pharma, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Spherix, Aria, Bioventus, Blackrock, Equilium, Glenmark, Horizon, Kypha, Lilly, MiMedx, Sorrento, Tonix, Priovant; E. Rusli, Scipher Medicine; L. Zhang, Scipher Medicine; C. Le-Short, Scipher Medicine Corporation; A. Arnaud, Scipher Medicine; J. Withers, Scipher Medicine Corporation; S. Asgarian, Scipher Medicine.

