Back
Poster Session B
Session: (0807–0832) Miscellaneous Rheumatic and Inflammatory Diseases Poster II
0812: 3-years Safety and Efficacy Outcomes of Canakinumab Treatment in Cryopyrin-associated Periodic Syndromes (CAPS) – Data from the RELIANCE Registry
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Jasmin Kümmerle-Deschner, MD
med.uni-tuebingen
Tübingen, Germany
Abstract Poster Presenter(s)
Jasmin B. Kuemmerle-Deschner1, Birgit Kortus-Goetze2, Prasad T. Oommen3, Ales Janda4, Juergen Rech5, Catharina Schuetz6, Tilmann Kallinich7, Frank Weller-Heinemann8, Gerd Horneff9, Ivan Foeldvari10, Florian Meier11, Michael Borte12, Tobias Krickau13, Julia Weber-Arden14 and Norbert Blank15, 1Department of Pediatrics, Division of Pediatric Rheumatology, University Hospital Tübingen, Tübingen, Germany, 2Division of Nephrology, University of Marburg, Marburg, Germany, 3Clinic of Pediatric Hematology, Oncology and Clinical Immunology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany, 4Department of Pediatrics, University Hospital Ulm, Ulm, Germany, 5University Clinic Erlangen, Erlangen, Germany, 6Pediatrics, Medizinische Fakultaet Carl Gustav Carus, Technische Universitaet Dresden, Dresden, Germany, 7Charité - Universitätsmedizin Berlin, Nuremberg, Germany, 8Klinikum Bremen-Mitte, Prof. Hess Kinderklinik, Bremen, Germany, 9Pediatrics, Asklepios Klinik Sankt Augustin GmbH, Sankt Augustin, Germany, 10Hamburger Zentrum für Kinder- und Jugendrheumatologie, Hamburg, Germany, 11Division of Rheumatology, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany, and Fraunhofer Institute for Molecular Biology and Applied Ecology IME, Project Group Translational Medicine and Pharmacology TMP, Frankfurt, Germany, 12ImmunoDeficiencyCenter Leipzig (IDCL), Hospital St. Georg gGmbH Leipzig, Germany, Leipzig, Sachsen, Germany, 13Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Pediatrics, Erlangen, Germany, 14Novartis Pharma GmbH, Nuernberg, Germany, 15Rheumatology, University Hospital Heidelberg, Heidelberg, Germany
Background/Purpose: Cryopyrin-associated periodic syndromes (CAPS) are monogenic autoinflammatory diseases with severe systemic inflammation. The IL-1β inhibitor canakinumab (CAN) leads to a rapid remission of CAPS symptoms in clinical trials as well as in practice. The RELIANCE registry investigates the long-term safety and efficacy of CAN under routine clinical conditions in pediatric (≥2 years) and adult patients with CAPS, including MWS, FCAS, and NOMID/CINCA (CINCA: chronic infantile neurologic cutaneous articular syndrome, FCAS: familial cold-induced autoinflammatory syndrome, MWS: muckle-wells syndrome, NOMID: neonatal multisystem inflammatory syndrome)
Methods: This prospective, non-interventional, observational study enrolls patients with a clinically confirmed diagnosis of CAPS who routinely receive CAN. Clinical data, physician assessments, and patient-reported outcomes will be collected at baseline and at 6-monthly visits.
Results: 98 CAPS patients (52% female; median age 20 years; median duration of prior CAN treatment 6 years) were enrolled in the study through December 2021. At the 36-months visit, both physicians and patients of all ages rated current disease activity as absent or mild/moderate (Table 1). While one-third of adult patients each received less than the standard dose, the standard dose, or a higher dose, the proportion of pediatric CAPS patients with higher doses was significantly greater ( < 12 years: 76%, 12-17 years: 43%). The proportion of patients without disease activity was highest in < 12-year-old patients (78%). Proportionate, more AE, SAE, and presumably drug-related SAE occurred in the pediatric cohort. Pathogenic mutations were documented for a total of N=38 patients, including R260W: N=15, A439V: N=9, T348M: N=9, D303N: N=3, and E627G, G755R, and G569R: N=1 each. Disease activity in these patients according to physician rating was absent and mild/moderate at a ratio of 1:1 (N=18:20). In contrast, this ratio was 2:1 in all other patients (N=38:19). Severe disease activity occurred in one patient with the V198M mutation. N=27 patients with pathogenic mutation received standard dose CAN and N=9 patients received higher dose.
Conclusion: The 36-months interim analysis of the RELIANCE study shows that long-term treatment with CAN is safe and effective in patients with CAPS regardless of the underlying mutation. Pediatric patients tend to have a higher infection rate with a better response rate.
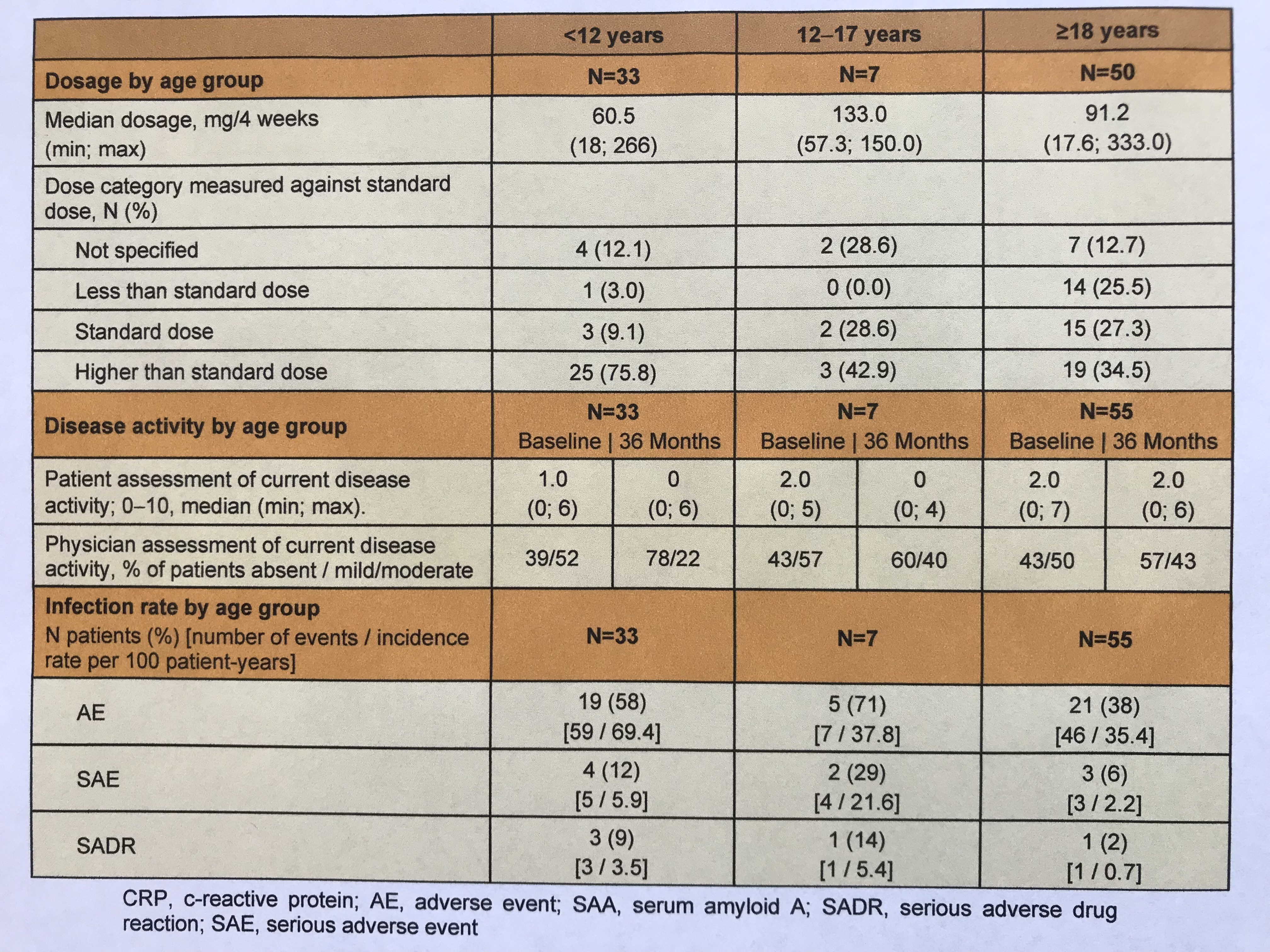 Table 1: Age-dependent dosing of canakinumab, assessment of clinical disease activity, and incidence of infection.
Table 1: Age-dependent dosing of canakinumab, assessment of clinical disease activity, and incidence of infection.
Disclosures: J. Kuemmerle-Deschner, AbbVie/Abbott, Novartis, SOBI; B. Kortus-Goetze, Novartis; P. Oommen, Novartis; A. Janda, None; J. Rech, Novartis, SOBI, AbbVie/Abbott, Biogen, Bristol-Myers Squibb(BMS), Chugai, GlaxoSmithKlein(GSK), Janssen, Eli Lilly, Merck/MSD, Mylan, Roche, Sanofi, UCB; C. Schuetz, Novartis; T. Kallinich, Roche; F. Weller-Heinemann, None; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott; I. Foeldvari, None; F. Meier, Novartis; M. Borte, Pfizer, Shire; T. Krickau, Novartis; J. Weber-Arden, Novartis; N. Blank, Novartis, SOBI, Lilly, Pfizer, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Actelion, UCB, Boehringer-Ingelheim, Roche.
Background/Purpose: Cryopyrin-associated periodic syndromes (CAPS) are monogenic autoinflammatory diseases with severe systemic inflammation. The IL-1β inhibitor canakinumab (CAN) leads to a rapid remission of CAPS symptoms in clinical trials as well as in practice. The RELIANCE registry investigates the long-term safety and efficacy of CAN under routine clinical conditions in pediatric (≥2 years) and adult patients with CAPS, including MWS, FCAS, and NOMID/CINCA (CINCA: chronic infantile neurologic cutaneous articular syndrome, FCAS: familial cold-induced autoinflammatory syndrome, MWS: muckle-wells syndrome, NOMID: neonatal multisystem inflammatory syndrome)
Methods: This prospective, non-interventional, observational study enrolls patients with a clinically confirmed diagnosis of CAPS who routinely receive CAN. Clinical data, physician assessments, and patient-reported outcomes will be collected at baseline and at 6-monthly visits.
Results: 98 CAPS patients (52% female; median age 20 years; median duration of prior CAN treatment 6 years) were enrolled in the study through December 2021. At the 36-months visit, both physicians and patients of all ages rated current disease activity as absent or mild/moderate (Table 1). While one-third of adult patients each received less than the standard dose, the standard dose, or a higher dose, the proportion of pediatric CAPS patients with higher doses was significantly greater ( < 12 years: 76%, 12-17 years: 43%). The proportion of patients without disease activity was highest in < 12-year-old patients (78%). Proportionate, more AE, SAE, and presumably drug-related SAE occurred in the pediatric cohort. Pathogenic mutations were documented for a total of N=38 patients, including R260W: N=15, A439V: N=9, T348M: N=9, D303N: N=3, and E627G, G755R, and G569R: N=1 each. Disease activity in these patients according to physician rating was absent and mild/moderate at a ratio of 1:1 (N=18:20). In contrast, this ratio was 2:1 in all other patients (N=38:19). Severe disease activity occurred in one patient with the V198M mutation. N=27 patients with pathogenic mutation received standard dose CAN and N=9 patients received higher dose.
Conclusion: The 36-months interim analysis of the RELIANCE study shows that long-term treatment with CAN is safe and effective in patients with CAPS regardless of the underlying mutation. Pediatric patients tend to have a higher infection rate with a better response rate.
 Table 1: Age-dependent dosing of canakinumab, assessment of clinical disease activity, and incidence of infection.
Table 1: Age-dependent dosing of canakinumab, assessment of clinical disease activity, and incidence of infection.Disclosures: J. Kuemmerle-Deschner, AbbVie/Abbott, Novartis, SOBI; B. Kortus-Goetze, Novartis; P. Oommen, Novartis; A. Janda, None; J. Rech, Novartis, SOBI, AbbVie/Abbott, Biogen, Bristol-Myers Squibb(BMS), Chugai, GlaxoSmithKlein(GSK), Janssen, Eli Lilly, Merck/MSD, Mylan, Roche, Sanofi, UCB; C. Schuetz, Novartis; T. Kallinich, Roche; F. Weller-Heinemann, None; G. Horneff, Roche, Pfizer, Novartis, Merck/MSD, Eli Lilly, AbbVie/Abbott; I. Foeldvari, None; F. Meier, Novartis; M. Borte, Pfizer, Shire; T. Krickau, Novartis; J. Weber-Arden, Novartis; N. Blank, Novartis, SOBI, Lilly, Pfizer, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Actelion, UCB, Boehringer-Ingelheim, Roche.

